Abstract
Background: Degenerative lumbar spinal stenosis (LSS) is usually caused by disc herniation or degeneration. Several genetic factors have been implicated in disc disease. Tryptophan alleles in COL9A2 and COL9A3 have been shown to be associated with lumbar disc disease in the Finnish population, and polymorphisms in the vitamin D receptor gene (VDR) (FokI and TaqI), the matrix metalloproteinase-3 gene (MMP-3) and an aggrecan gene (AGC1) VNTR have been reported to be associated with disc degeneration. In addition, an IVS6-4 a>t polymorphism in COL11A2 has been found in connection with stenosis caused by ossification of the posterior longitudinal ligament in the Japanese population.
Objective: To study the role of genetic factors in LSS.
Methods: 29 Finnish probands were analysed for mutations in the genes coding for intervertebral disc matrix proteins, COL1A1, COL1A2, COL2A1, COL9A1, COL9A2, COL9A3, COL11A1, COL11A2, and AGC1. VDR and MMP-3 polymorphisms were also analysed. Sequence variations were tested in 56 Finnish controls.
Results: Several disease associated alleles were identified. A splice site mutation in COL9A2 leading to a premature translation termination codon and the generation of a truncated protein was identified in one proband, another had the Trp2 allele, and four others the Trp3 allele. The frequency of the COL11A2 IVS6-4 t allele was 93.1% in the probands and 72.3% in controls (p = 0.0016). The differences in genotype frequencies for this site were less significant (p = 0.0043).
Conclusions: Genetic factors have an important role in the pathogenesis of LSS.
Full Text
The Full Text of this article is available as a PDF (361.4 KB).
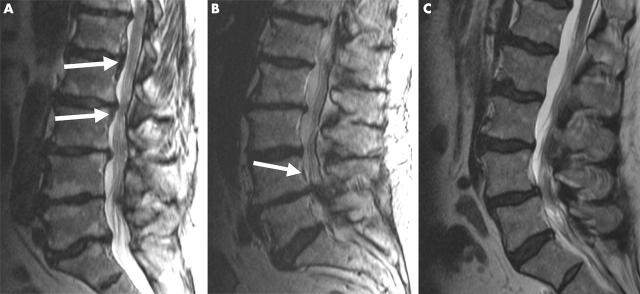
Figure 1 .
T2 weighted transaxial MRI scans (TR 6000 ms/TE 105 ms) of probands 2 (A), 10 (B), and 23 (C). (A) A hypertrophic band is seen at the L2–3 disc level and caudally at L3–4 (white arrows). Both disc and end plate degeneration are seen at all lumbar levels. (B) The scan shows OPLL at the L4 vertebral level (white arrow). This proband also had severe disc and end plate degeneration at multiple lumbar levels. (C) The scan indicates severe disc degeneration at multiple lumbar levels, and a Schmorl's node at L2–3.
Figure 2 .
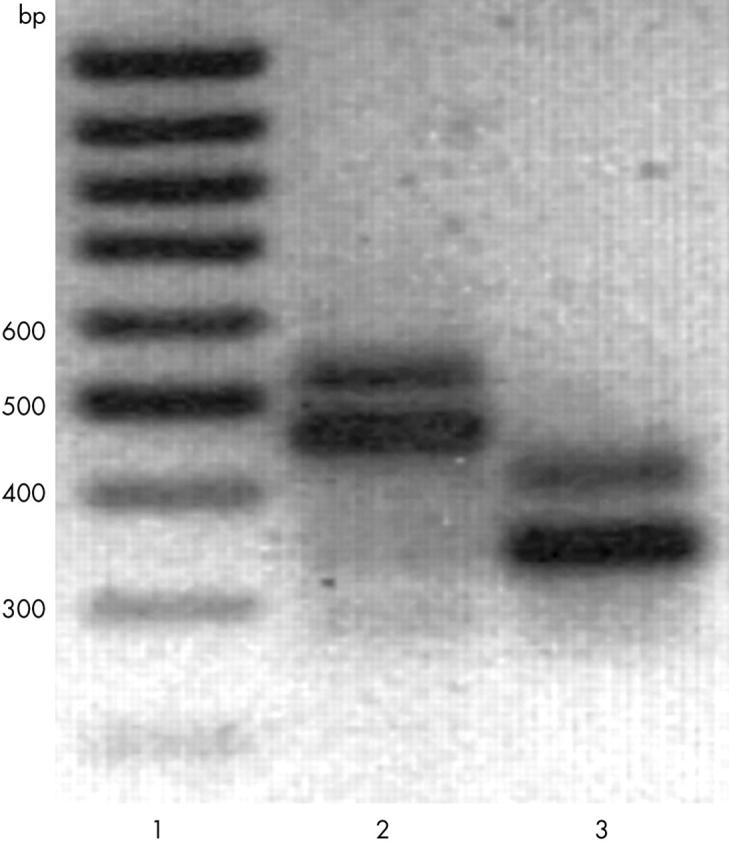
Agarose gel electrophoresis of α2(IX) RT-PCR products. Total RNA from proband 23 was analysed by RT-PCR using primer pairs corresponding to exons 23 and 30 (lane 2), or exons 24 and 29 (lane 3), as indicated in "Materials and methods". The analysis showed two products of about 550 bp and 460 bp (lane 2) and 420 bp and 340 bp (lane 3). Sequencing indicated that the lower molecular weight products contained the wild-type sequence, whereas the higher molecular weight products also contained intron 26. Lane 1 contains a 100 bp ladder.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kvist A. P., Metsäranta M., Kivirikko K. I., de Crombrugghe B., Prockop D. J., Vuorio E. Conservation of the sizes of 53 introns and over 100 intronic sequences for the binding of common transcription factors in the human and mouse genes for type II procollagen (COL2A1). Biochem J. 1995 Jun 15;308(Pt 3):923–929. doi: 10.1042/bj3080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko Leena. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34(1):42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- Annunen S., Körkkö J., Czarny M., Warman M. L., Brunner H. G., Käriäinen H., Mulliken J. B., Tranebjaerg L., Brooks D. G., Cox G. F. Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes. Am J Hum Genet. 1999 Oct;65(4):974–983. doi: 10.1086/302585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunen S., Paassilta P., Lohiniva J., Perälä M., Pihlajamaa T., Karppinen J., Tervonen O., Kröger H., Lähde S., Vanharanta H. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999 Jul 16;285(5426):409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- Arnoldi C. C., Brodsky A. E., Cauchoix J., Crock H. V., Dommisse G. F., Edgar M. A., Gargano F. P., Jacobson R. E., Kirkaldy-Willis W. H., Kurihara A. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976 Mar-Apr;(115):4–5. [PubMed] [Google Scholar]
- Cartegni Luca, Chew Shern L., Krainer Adrian R. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet. 2002 Apr;3(4):285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- Chiano M. N., Clayton D. G. Fine genetic mapping using haplotype analysis and the missing data problem. Ann Hum Genet. 1998 Jan;62(Pt 1):55–60. doi: 10.1046/j.1469-1809.1998.6210055.x. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Coulter S. N., Meek L. M., Maslen K., Wood J. G. A human-specific polymorphism in the coding region of the aggrecan gene. Variable number of tandem repeats produce a range of core protein sizes in the general population. J Biol Chem. 1997 May 23;272(21):13974–13979. doi: 10.1074/jbc.272.21.13974. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Gross C., Eccleshall T. R., Malloy P. J., Villa M. L., Marcus R., Feldman D. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican-American women. J Bone Miner Res. 1996 Dec;11(12):1850–1855. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- Hall S., Bartleson J. D., Onofrio B. M., Baker H. L., Jr, Okazaki H., O'Duffy J. D. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985 Aug;103(2):271–275. doi: 10.7326/0003-4819-103-2-271. [DOI] [PubMed] [Google Scholar]
- Harris S. S., Eccleshall T. R., Gross C., Dawson-Hughes B., Feldman D. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997 Jul;12(7):1043–1048. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- Jones G., White C., Sambrook P., Eisman J. Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann Rheum Dis. 1998 Feb;57(2):94–99. doi: 10.1136/ard.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Osada R., Kanamori M., Ishihara H., Ohmori K., Matsui H., Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine (Phila Pa 1976) 1999 Dec 1;24(23):2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- Kimura T., Nakata K., Tsumaki N., Miyamoto S., Matsui Y., Ebara S., Ochi T. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int Orthop. 1996;20(3):177–181. doi: 10.1007/s002640050058. [DOI] [PubMed] [Google Scholar]
- Koga H., Sakou T., Taketomi E., Hayashi K., Numasawa T., Harata S., Yone K., Matsunaga S., Otterud B., Inoue I. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998 Jun;62(6):1460–1467. doi: 10.1086/301868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körkkö J., Ala-Kokko L., De Paepe A., Nuytinck L., Earley J., Prockop D. J. Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation-sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: identification of common sequences of null-allele mutations. Am J Hum Genet. 1998 Jan;62(1):98–110. doi: 10.1086/301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körkkö J., Annunen S., Pihlajamaa T., Prockop D. J., Ala-Kokko L. Conformation sensitive gel electrophoresis for simple and accurate detection of mutations: comparison with denaturing gradient gel electrophoresis and nucleotide sequencing. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1681–1685. doi: 10.1073/pnas.95.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Ishidou Y., Koga H., Taketomi E., Ikari K., Komiya S., Takeda J., Sakou T., Inoue I. Functional impact of human collagen alpha2(XI) gene polymorphism in pathogenesis of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2001 May;16(5):948–957. doi: 10.1359/jbmr.2001.16.5.948. [DOI] [PubMed] [Google Scholar]
- Modic M. T., Steinberg P. M., Ross J. S., Masaryk T. J., Carter J. R. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988 Jan;166(1 Pt 1):193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Qi J. C., Tokita A., Kelly P. J., Crofts L., Nguyen T. V., Sambrook P. N., Eisman J. A. Prediction of bone density from vitamin D receptor alleles. Nature. 1994 Jan 20;367(6460):284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- Myllyharju J., Kivirikko K. I. Collagens and collagen-related diseases. Ann Med. 2001 Feb;33(1):7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- Nakata K., Ono K., Miyazaki J., Olsen B. R., Muragaki Y., Adachi E., Yamamura K., Kimura T. Osteoarthritis associated with mild chondrodysplasia in transgenic mice expressing alpha 1(IX) collagen chains with a central deletion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2870–2874. doi: 10.1073/pnas.90.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasawa T., Koga H., Ueyama K., Maeda S., Sakou T., Harata S., Leppert M., Inoue I. Human retinoic X receptor beta: complete genomic sequence and mutation search for ossification of posterior longitudinal ligament of the spine. J Bone Miner Res. 1999 Apr;14(4):500–508. doi: 10.1359/jbmr.1999.14.4.500. [DOI] [PubMed] [Google Scholar]
- Paassilta P., Lohiniva J., Göring H. H., Perälä M., Räinä S. S., Karppinen J., Hakala M., Palm T., Kröger H., Kaitila I. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001 Apr 11;285(14):1843–1849. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- Paassilta P., Pihlajamaa T., Annunen S., Brewton R. G., Wood B. M., Johnson C. C., Liu J., Gong Y., Warman M. L., Prockop D. J. Complete sequence of the 23-kilobase human COL9A3 gene. Detection of Gly-X-Y triplet deletions that represent neutral variants. J Biol Chem. 1999 Aug 6;274(32):22469–22475. doi: 10.1074/jbc.274.32.22469. [DOI] [PubMed] [Google Scholar]
- Pihlajamaa T., Vuoristo M. M., Annunen S., Perälä M., Prockop D. J., Ala-Kokko L. Human COL9A1 and COL9A2 genes. Two genes of 90 and 15 kb code for similar polypeptides of the same collagen molecule. Matrix Biol. 1998 Jul;17(3):237–241. doi: 10.1016/s0945-053x(98)90063-4. [DOI] [PubMed] [Google Scholar]
- Porter R. W. Spinal stenosis and neurogenic claudication. Spine (Phila Pa 1976) 1996 Sep 1;21(17):2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- Saijo T., Ito M., Takeda E., Huq A. H., Naito E., Yokota I., Sone T., Pike J. W., Kuroda Y. A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: utility of single-strand conformation polymorphism analysis for heterozygous carrier detection. Am J Hum Genet. 1991 Sep;49(3):668–673. [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. M., Shaffrey C. I., Jane J. A., Sr Degenerative lumbar stenosis: the neurosurgical perspective. Clin Orthop Relat Res. 2001 Mar;(384):61–74. [PubMed] [Google Scholar]
- Spengler D. M. Degenerative stenosis of the lumbar spine. J Bone Joint Surg Am. 1987 Feb;69(2):305–308. [PubMed] [Google Scholar]
- Spivak J. M. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998 Jul;80(7):1053–1066. doi: 10.2106/00004623-199807000-00015. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Haro H., Wakabayashi Y., Kawa-uchi T., Komori H., Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001 May;83(4):491–495. doi: 10.1302/0301-620x.83b4.11617. [DOI] [PubMed] [Google Scholar]
- Valhmu W. B., Palmer G. D., Rivers P. A., Ebara S., Cheng J. F., Fischer S., Ratcliffe A. Structure of the human aggrecan gene: exon-intron organization and association with the protein domains. Biochem J. 1995 Jul 15;309(Pt 2):535–542. doi: 10.1042/bj3090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varughese G., Quartey G. R. Familial lumbar spinal stenosis with acute disc herniations. Case reports of four brothers. J Neurosurg. 1979 Aug;51(2):234–236. doi: 10.3171/jns.1979.51.2.0234. [DOI] [PubMed] [Google Scholar]
- Videman T., Gibbons L. E., Battié M. C., Maravilla K., Vanninen E., Leppävuori J., Kaprio J., Peltonen L. The relative roles of intragenic polymorphisms of the vitamin d receptor gene in lumbar spine degeneration and bone density. Spine (Phila Pa 1976) 2001 Feb 1;26(3):E7–E12. doi: 10.1097/00007632-200102010-00003. [DOI] [PubMed] [Google Scholar]
- Videman T., Leppävuori J., Kaprio J., Battié M. C., Gibbons L. E., Peltonen L., Koskenvuo M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine (Phila Pa 1976) 1998 Dec 1;23(23):2477–2485. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- Vuristo M. M., Pihlajamaa T., Vandenberg P., Prockop D. J., Ala-Kokko L. The human COL11A2 gene structure indicates that the gene has not evolved with the genes for the major fibrillar collagens. J Biol Chem. 1995 Sep 29;270(39):22873–22881. doi: 10.1074/jbc.270.39.22873. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Nakata K., Kimata K., Nakanishi I., Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6943–6947. doi: 10.1073/pnas.94.13.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Davies R., Walsh W. K., Gormley J. Achondroplasia and hypochondroplasia. Clinical variation and spinal stenosis. J Bone Joint Surg Br. 1981;63B(4):508–515. doi: 10.1302/0301-620X.63B4.7298674. [DOI] [PubMed] [Google Scholar]
- Ye S., Watts G. F., Mandalia S., Humphries S. E., Henney A. M. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J. 1995 Mar;73(3):209–215. doi: 10.1136/hrt.73.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]