Abstract
Methods: Eleven patients from the European multicentre trial of the efficacy and safety of etanercept were included in this study when transferred into the open label, long term safety, and efficacy study. Before a scheduled dosage increase to 50 mg/week they were examined clinically, serologically, and by ultrasonography using the colour Doppler pixels and the spectral Doppler resistance index (RI) as indicators of inflammation. The patients were re-examined at two weeks and at one year follow up
Results: The clinical activity decreased significantly from baseline to week 2, but no significant changes were seen from baseline to one year. The number of coloured pixels in each region of interest decreased from baseline to week 2 with a median reduction of 60% (p=0.005). This effect on the perfusion in the synovium could not be found after one year of treatment. During the initial treatment we detected an increase in synovial RI by spectral Doppler. The median increase in peripheral resistance from baseline to week 2 as estimated by the mean RI was 22.6% (p=0.005). The increase in peripheral resistance was maintained to some extent after one year (mean RI increased by 18.8% p=0.074).
Conclusion: Ultrasonography seems to be a promising tool for the detection of treatment response using spectral Doppler and pixel estimation.
Full Text
The Full Text of this article is available as a PDF (188.6 KB).
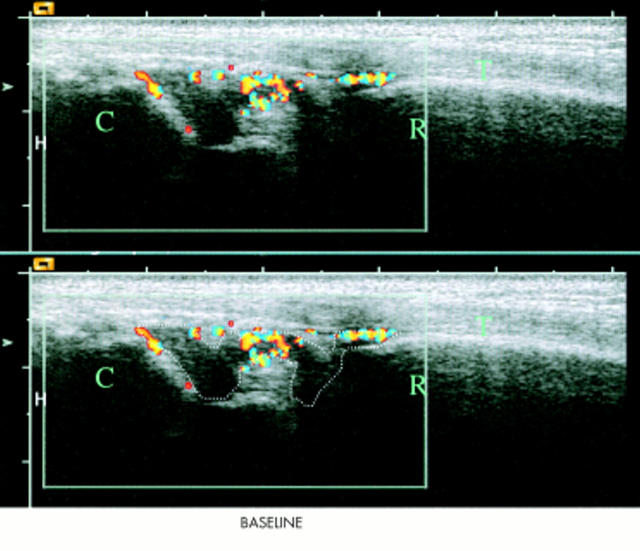
Figure 1.
Longitudinal scan of the right wrist at baseline with the radial bone marked as R, the carpus marked as C, and the extensor digitorum tendon marked as T. The hypervascular nature of the synovium is illustrated with colour Doppler and the region of interest (the hypertrophic synovium) is outlined. A false appearance of flow inside the bone is due to an artefact.
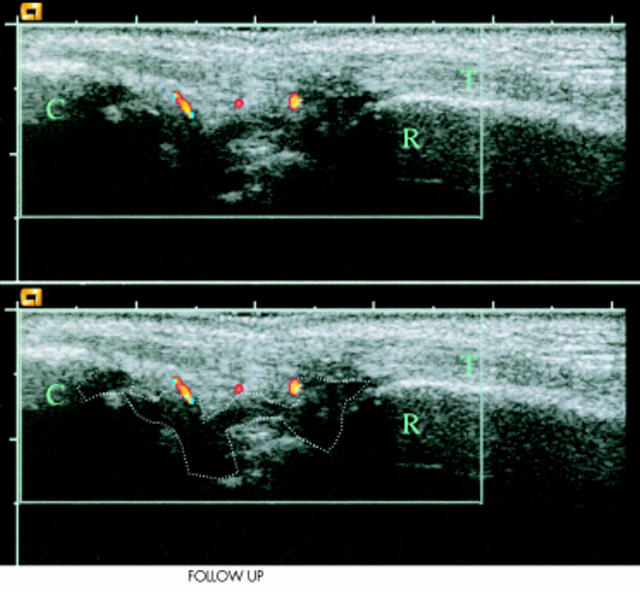
Figure 2.
Longitudinal scan of the right wrist at week 2 with the radial bone marked as R, the carpus marked as C, and the extensor digitorum tendon marked as T. The hypervascular nature of the synovium is illustrated with colour Doppler and the region of interest (the hypertrophic synovium) is outlined. Compared with the baseline examination, there is a marked decline in Doppler activity at week 2 follow up and most of Doppler activity found is localised outside the hypertrophic synovium, and is not used in our measurements.