Abstract
Background: Recent studies have shown that adipose tissue is an endocrine organ that releases various cytokines.
Objective: To investigate the production of growth factors and proinflammatory cytokines in infrapatellar fat pad specimens.
Methods: Infrapatellar fat pad tissues were obtained from patients during knee surgery. Protein levels of basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), tumour necrosis factor (TNF)α, and interleukin (IL)6 in homogenised tissues were measured by an enzyme immunoassay. Gene expressions for those cytokines were examined by reverse transcription-polymerase chain reaction (RT-PCR). Localisation of bFGF and VEGF was evaluated by immunohistochemistry and in situ hybridisation.
Results: Infrapatellar fat pads were found to contain various protein levels of bFGF, VEGF, TNFα, and IL6. Further, gene expressions for these cytokines were detected by RT-PCR. Immunohistochemistry and in situ hybridisation showed that the expressions of both bFGF and VEGF were localised in immature adipocytes, interstitial undifferentiated mesenchymal cells, and vascular endothelial cells.
Conclusion: The production of bFGF, VEGF, TNFα, and IL6 in the infrapatellar fat pad was demonstrated. Although synovial cells and articular chondrocytes are thought to be primary sources of cytokines found in knee synovial fluids, the results suggest that they may also originate from this fat pad.
Full Text
The Full Text of this article is available as a PDF (166.4 KB).
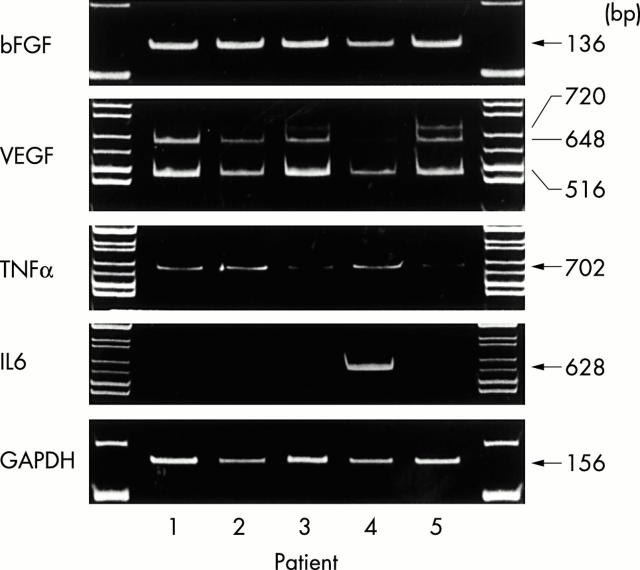
Figure 1.
RT-PCR results for bFGF, VEGF, TNFα, IL6, and GAPDH in infrapatellar fat pad tissues. Expected sizes of the amplified DNA fragments are shown in the ethidium bromide 6% polyacrylamide gel electrophoresis images. The number under each panel indicates patient number, whose characteristics are shown in table 1. Amplified fragments of bFGF, the lower three VEGF isoforms (VEGF121: 516 bp, VEGF165: 648 bp, VEGF189: 720 bp), and TNFα were detected in all five specimens, whereas those of IL6 were detected in only one.
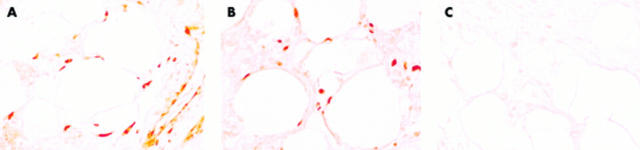
Figure 2.
Immunohistochemical staining of infrapatellar fat pad tissues. (A) bFGF, (B) VEGF, (C) normal rabbit IgG. Positive cytoplasmic staining for bFGF and VEGF was detected in relatively immature adipocytes, interstitial undifferentiated mesenchymal cells, and vascular endothelial cells. Original magnification x100.
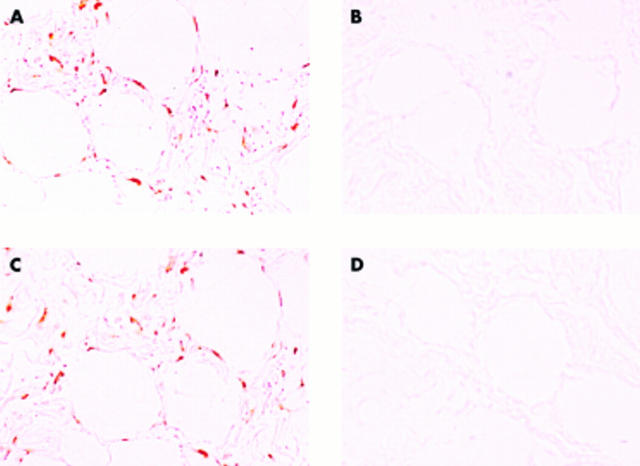
Figure 3.
In situ hybridisation results for bFGF and VEGF in infrapatellar fat pad tissues. (A) Anti-sense probe for bFGF, (B) sense probe for bFGF, (C) anti-sense probe for VEGF, (D) sense probe for VEGF. Positive signals for bFGF and VEGF were detected in the cytoplasm of relatively immature adipocytes, interstitial undifferentiated mesenchymal cells, and vascular endothelial cells. Original magnification x50.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomley M. J., Webb N. J., Watson C. J., Holt L., Bukhari M., Denton J., Freemont A. J., Brenchley P. E. Placenta growth factor (PlGF) induces vascular endothelial growth factor (VEGF) secretion from mononuclear cells and is co-expressed with VEGF in synovial fluid. Clin Exp Immunol. 2000 Jan;119(1):182–188. doi: 10.1046/j.1365-2249.2000.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butch A. W., Chung G. H., Hoffmann J. W., Nahm M. H. Cytokine expression by germinal center cells. J Immunol. 1993 Jan 1;150(1):39–47. [PubMed] [Google Scholar]
- Cameron M. L., Fu F. H., Paessler H. H., Schneider M., Evans C. H. Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deficient knee. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):38–44. doi: 10.1007/BF01552652. [DOI] [PubMed] [Google Scholar]
- Carlevaro M. F., Cermelli S., Cancedda R., Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000 Jan;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- Claffey K. P., Wilkison W. O., Spiegelman B. M. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992 Aug 15;267(23):16317–16322. [PubMed] [Google Scholar]
- Cronauer M. V., Stadlmann S., Klocker H., Abendstein B., Eder I. E., Rogatsch H., Zeimet A. G., Marth C., Offner F. A. Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. Am J Pathol. 1999 Dec;155(6):1977–1984. doi: 10.1016/S0002-9440(10)65516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B., Padró T., Leo R., Feldmann B., Kropff M., Mesters R. M., Serve H., Berdel W. E., Kienast J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000 Apr 15;95(8):2630–2636. [PubMed] [Google Scholar]
- Felson D. T. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996 Sep;55(9):668–670. doi: 10.1136/ard.55.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D. T., Lawrence R. C., Dieppe P. A., Hirsch R., Helmick C. G., Jordan J. M., Kington R. S., Lane N. E., Nevitt M. C., Zhang Y. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000 Oct 17;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Vu T. H., Ryan A. M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999 Jun;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Hart D. J., Doyle D. V., Spector T. D. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995 Jun;22(6):1118–1123. [PubMed] [Google Scholar]
- Hochberg M. C., Lethbridge-Cejku M., Scott W. W., Jr, Reichle R., Plato C. C., Tobin J. D. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1995 Mar;22(3):488–493. [PubMed] [Google Scholar]
- Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995 May;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Hosoda Y., Hirose S., Okada Y., Ikeda E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000 Aug;191(4):426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Magi M., Branca A., Bucca C., Langerame V. Hoffa disease. Ital J Orthop Traumatol. 1991 Jun;17(2):211–216. [PubMed] [Google Scholar]
- Manicourt D. H., Poilvache P., Van Egeren A., Devogelaer J. P., Lenz M. E., Thonar E. J. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000 Feb;43(2):281–288. doi: 10.1002/1529-0131(200002)43:2<281::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A., Katz D. R., Miles J. M., Yudkin J. S., Klein S., Coppack S. W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997 Dec;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V., Pinkney J. H., Coppack S. W. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998 Dec;22(12):1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- Posever J., Phillips F. M., Pottenger L. A. Effects of basic fibroblast growth factor, transforming growth factor-beta 1, insulin-like growth factor-1, and insulin on human osteoarthritic articular cartilage explants. J Orthop Res. 1995 Nov;13(6):832–837. doi: 10.1002/jor.1100130605. [DOI] [PubMed] [Google Scholar]
- Pufe T., Petersen W., Tillmann B., Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001 May;44(5):1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Qu Z., Huang X. N., Ahmadi P., Andresevic J., Planck S. R., Hart C. E., Rosenbaum J. T. Expression of basic fibroblast growth factor in synovial tissue from patients with rheumatoid arthritis and degenerative joint disease. Lab Invest. 1995 Sep;73(3):339–346. [PubMed] [Google Scholar]
- Schlaak J. F., Schwarting A., Knolle P., Meyer zum Büschenfelde K. H., Mayet W. Effects of Th1 and Th2 cytokines on cytokine production and ICAM-1 expression on synovial fibroblasts. Ann Rheum Dis. 1995 Jul;54(7):560–565. doi: 10.1136/ard.54.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P. Z., Gannon M. J., Andrew A., Miller D. Evidence for oestrogenic regulation of heat shock protein expression in human endometrium and steroid-responsive cell lines. Eur J Endocrinol. 1995 Nov;133(5):598–605. doi: 10.1530/eje.0.1330598. [DOI] [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Geng C., Cloutier J. M., Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999 Jun;42(6):1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Uchino M., Izumi T., Tominaga T., Wakita R., Minehara H., Sekiguchi M., Itoman M. Growth factor expression in the osteophytes of the human femoral head in osteoarthritis. Clin Orthop Relat Res. 2000 Aug;(377):119–125. doi: 10.1097/00003086-200008000-00017. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Oh-ishi S., Kizaki T., Nagasawa J., Saitoh D., Ohira Y., Ohno H. Insulin stimulates the expression of basic fibroblast growth factor in rat brown adipocyte primary culture. Eur J Cell Biol. 1995 Sep;68(1):8–13. [PubMed] [Google Scholar]