Abstract
Background: Increased levels of B lymphocyte stimulator (BLyS) have been detected in serum from patients with systemic lupus erythematosus and rheumatoid arthritis.
Objective: To determine the level of BLyS in serum from patients with primary's Sjögren's syndrome (SS), another autoimmune disease in which B cell activation is high.
Methods: Serum samples from 49 patients with primary SS according to the revised European criteria were assayed for BLyS, quantitative immunoglobulins, and autoantibody levels and compared with samples from 47 healthy control subjects.
Results: The median level of BLyS was 5.99 ng/ml (25th-75th centile range 3.20–8.93 ng/ml) in SS v 2.49 ng/ml (25th-75th centile range 1.96–2.96 ng/ml) in healthy controls (p<0.001). More importantly, among patients with SS, the presence of anti-SSA antibodies was associated with significantly higher levels of BLyS (medians 7.90 ng/ml v 3.70 ng/ml; p=0.008) as was the presence of anti-SSB antibodies (medians 7.14 ng/ml v 3.70 ng/ml; p=0.02) and of rheumatoid factor (medians 7.70 ng/ml v 3.80 ng/ml; p=0.016). The level of BLyS in three patients with a monoclonal gammopathy was higher than in the other patients (medians 26.53 ng/ml v 5.92 ng/ml; p=0.13). Higher levels of BLyS were associated with higher levels of gammaglobulins and IgG. There was a strong correlation between BLyS and rheumatoid factor level (r=0.71, p<0.0001), anti-SSA IgG level (r=0.32, p=0.02) and anti-SSA IgM level (r=0.39, p=0.006).
Conclusion: In human SS the level of BLyS correlates with the level of autoantibodies. Thus, BLyS may play a part in activating specific autoreactive B cells and modulating the level of production of autoantibodies which are the hallmark of the disease. These findings raise the possibility of a novel therapeutic approach in human SS.
Full Text
The Full Text of this article is available as a PDF (189.8 KB).
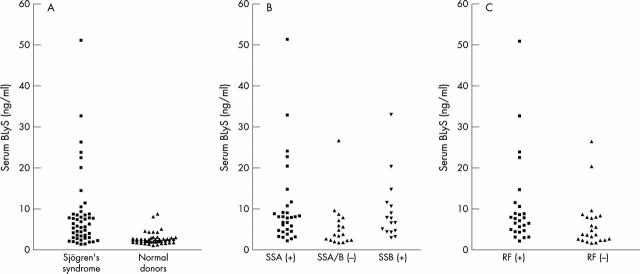
Figure 1.
BLyS levels in patients with SS. (A) Serum levels of BLyS in the patients with SS (n=49) were significantly higher than in normal donors (n=47), p<0.001. (B and C) Among patients with SS, BLyS levels were higher in patients with anti-SSA antibodies, anti-SSB antibodies, and in patients with RF (p=0.008, p=0.02, and p=0.016, respectively; Mann-Whitney test). The medians of each group were: normal, 2.49 ng/ml; SS, 5.99 ng/ml; SS with anti-SSA, 7.90 ng/ml; SS with anti-SSB, 7.14 ng/ml; SS without anti-SSA/SSB, 3.70 ng/ml; SS with RF, 7.70 ng/ml; SS without RF, 3.80 ng/ml.
Figure 2.

Correlation between BLyS and immunoglobulins. Correlations between serum levels of BLyS and serum gammaglobulin (A), and IgG (B) were determined for the 46 patients with SS without a monoclonal component. Analysis was based on log10 transformed values. The correlation coefficient was determined by the Pearson product statistic and the regression line is represented by the solid line.
Figure 3.

Correlation between BLyS and autoantibodies. Correlations between serum levels of BLyS and anti-SSA IgG (A), anti-SSA IgM (B) and serum RF (C) were determined by log10 transformed values. The correlation coefficient was determined by the Pearson product statistic and the regression line is represented by the solid line.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheema G. S., Roschke V., Hilbert D. M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001 Jun;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Groom Joanna, Kalled Susan L., Cutler Anne H., Olson Carl, Woodcock Stephen A., Schneider Pascal, Tschopp Jurg, Cachero Teresa G., Batten Marcel, Wheway Julie. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002 Jan;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. A., Johnston J., Mudri S., Enselman R., Dillon S. R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000 Apr 27;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- Khare S. D., Sarosi I., Xia X. Z., McCabe S., Miner K., Solovyev I., Hawkins N., Kelley M., Chang D., Van G. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korganow A. S., Ji H., Mangialaio S., Duchatelle V., Pelanda R., Martin T., Degott C., Kikutani H., Rajewsky K., Pasquali J. L. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999 Apr;10(4):451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- Laâbi Y., Gras M. P., Carbonnel F., Brouet J. C., Berger R., Larsen C. J., Tsapis A. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992 Nov;11(11):3897–3904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Woodcock S. A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J. L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999 Dec 6;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X. Lymphomas complicating Sjögren's syndrome and hepatitis C virus infection may share a common pathogenesis: chronic stimulation of rheumatoid factor B cells. Ann Rheum Dis. 2001 Nov;60(11):1007–1010. doi: 10.1136/ard.60.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I., Staub A., Benoist C., Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999 Nov 26;286(5445):1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- Moore P. A., Belvedere O., Orr A., Pieri K., LaFleur D. W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999 Jul 9;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Nose M., Sasano M., Kawashima Y. Salazosulfapyridine suppresses chondrocyte mediated degradation induced by interleukin 1beta. J Rheumatol. 1997 Mar;24(3):550–554. [PubMed] [Google Scholar]
- Schaller M., Burton D. R., Ditzel H. J. Autoantibodies to GPI in rheumatoid arthritis: linkage between an animal model and human disease. Nat Immunol. 2001 Aug;2(8):746–753. doi: 10.1038/90696. [DOI] [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J. L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999 Jun 7;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H. B., Hu W. H., Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999 May;65(5):680–683. [PubMed] [Google Scholar]
- Thompson J. S., Bixler S. A., Qian F., Vora K., Scott M. L., Cachero T. G., Hession C., Schneider P., Sizing I. D., Mullen C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001 Aug 16;293(5537):2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Marsters S. A., Baker T., Chan B., Lee W. P., Fu L., Tumas D., Yan M., Dixit V. M., Ashkenazi A. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol. 2001 Jul;2(7):632–637. doi: 10.1038/89782. [DOI] [PubMed] [Google Scholar]
- Zhang J., Roschke V., Baker K. P., Wang Z., Alarcón G. S., Fessler B. J., Bastian H., Kimberly R. P., Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001 Jan 1;166(1):6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]