Abstract
Objective: To investigate whether the recently developed (statistically derived) "ASsessment in Ankylosing Spondylitis Working Group" improvement criteria (ASAS-IC) for ankylosing spondylitis (AS) reflect clinically relevant improvement according to the opinion of an expert panel.
Methods: The ASAS-IC consist of four domains: physical function, spinal pain, patient global assessment, and inflammation. Scores on these four domains of 55 patients with AS, who had participated in a non-steroidal anti-inflammatory drug efficacy trial, were presented to an international expert panel (consisting of patients with AS and members of the ASAS Working Group) in a three round Delphi exercise. The number of (non-)responders according to the ASAS-IC was compared with the final consensus of the experts. The most important domains in the opinion of the experts were identified, and also selected with discriminant analysis. A number of provisional criteria sets that best represented the consensus of the experts were defined. Using other datasets, these clinically derived criteria sets as well as the statistically derived ASAS-IC were then tested for discriminative properties and for agreement with the end of trial efficacy by patient and doctor.
Results: Forty experts completed the three Delphi rounds. The experts considered twice as many patients to be responders than the ASAS-IC (42 v 21). Overall agreement between experts and ASAS-IC was 62%. Spinal pain was considered the most important domain by most experts and was also selected as such by discriminant analysis. Provisional criteria sets with an agreement of ⩾80% compared with the consensus of the experts showed high placebo response rates (27–42%), in contrast with the ASAS-IC with a predefined placebo response rate of 25%. All criteria sets and the ASAS-IC discriminated well between active and placebo treatment (χ2=36–45; p<0.001). Compared with the end of trial efficacy assessment, the provisional criteria sets showed an agreement of 71–82%, sensitivity of 67–83%, and specificity of 81–88%. The ASAS-IC showed an agreement of 70%, sensitivity of 62%, and specificity of 89%.
Conclusion: The ASAS-IC are strict in defining response, are highly specific, and consequently show lower sensitivity than the clinically derived criteria sets. However, those patients who are considered as responders by applying the ASAS-IC are acknowledged as such by the expert panel as well as by patients' and doctors' judgments, and are therefore likely to be true responders.
Full Text
The Full Text of this article is available as a PDF (206.5 KB).
Figure 1.
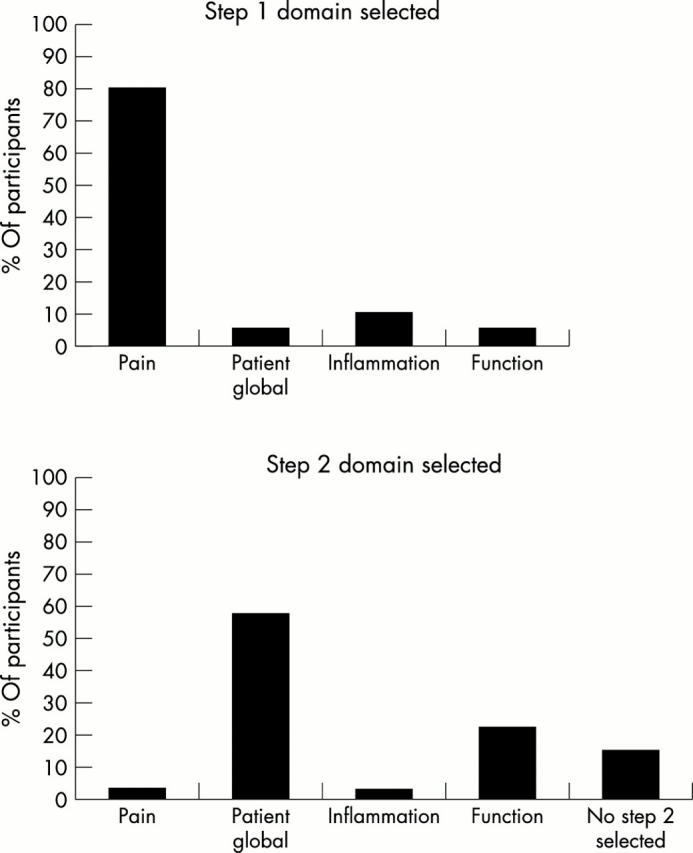
The percentage of participants in each domain selected as the first and second step in the discriminant analysis on the absolute change scores.
Figure 2.
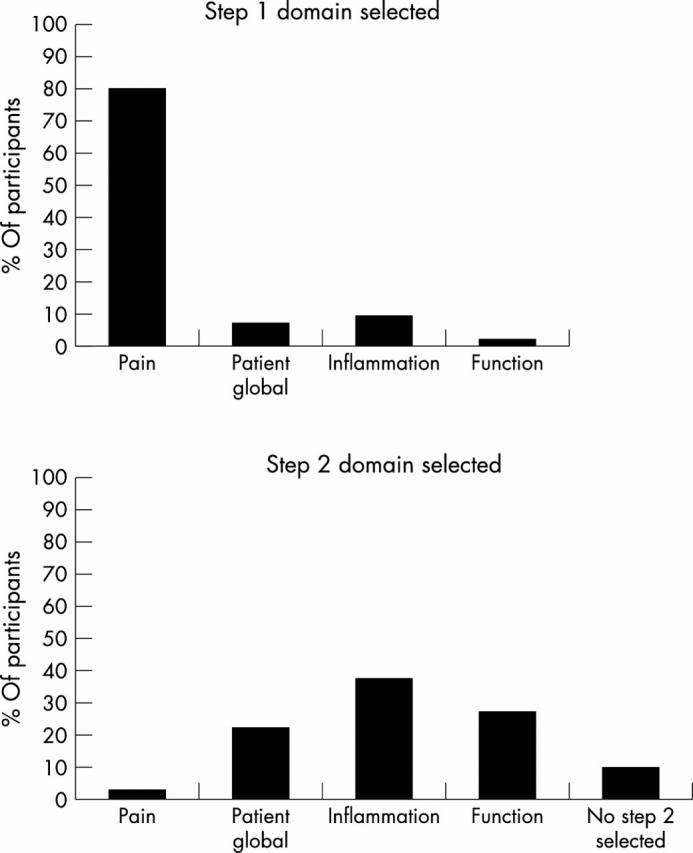
The percentage of participants in each domain selected as the first and second step in the discriminant analysis on the relative change scores.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Baron G., van der Heijde D., Felson D. T., Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001 Aug;44(8):1876–1886. doi: 10.1002/1529-0131(200108)44:8<1876::AID-ART326>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Calin A., Garrett S., Whitelock H., Kennedy L. G., O'Hea J., Mallorie P., Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994 Dec;21(12):2281–2285. [PubMed] [Google Scholar]
- Dougados M., Gueguen A., Nakache J. P., Velicitat P., Veys E. M., Zeidler H., Calin A. Ankylosing spondylitis: what is the optimum duration of a clinical study? A one year versus a 6 weeks non-steroidal anti-inflammatory drug trial. Rheumatology (Oxford) 1999 Mar;38(3):235–244. doi: 10.1093/rheumatology/38.3.235. [DOI] [PubMed] [Google Scholar]
- Dougados M., Leclaire P., van der Heijde D., Bloch D. A., Bellamy N., Altman R. D. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000 Nov;8(6):395–403. doi: 10.1053/joca.2000.0361. [DOI] [PubMed] [Google Scholar]
- Garrett S., Jenkinson T., Kennedy L. G., Whitelock H., Gaisford P., Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994 Dec;21(12):2286–2291. [PubMed] [Google Scholar]
- Giannini E. H., Ruperto N., Ravelli A., Lovell D. J., Felson D. T., Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997 Jul;40(7):1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995 Aug 5;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan J. R., Chaput de Saintonge D. M., Joyce C. R., Currey H. L. Clinical judgment in rheumatoid arthritis. II. Judging 'current disease activity' in clinical practice. Ann Rheum Dis. 1983 Dec;42(6):648–651. doi: 10.1136/ard.42.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Lennox J. A., McLaughlin-Miley C., Lennox R. D., Bohlig A. M., Cutler B. L., Yan C., Jaffe M. Stratification of flare intensity identifies placebo responders in a treatment efficacy trial of patients with osteoarthritis. Arthritis Rheum. 2001 Jul;44(7):1599–1607. doi: 10.1002/1529-0131(200107)44:7<1599::AID-ART283>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ward M. M. Response criteria and criteria for clinically important improvement: separate and equal? Arthritis Rheum. 2001 Aug;44(8):1728–1729. doi: 10.1002/1529-0131(200108)44:8<1728::AID-ART306>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- van Riel P. L., van de Putte L. B. DC-ART: what proportion of response constitutes a positive response? J Rheumatol Suppl. 1994 Sep;41:54–56. [PubMed] [Google Scholar]
- van der Heijde D., Calin A., Dougados M., Khan M. A., van der Linden S., Bellamy N. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol. 1999 Apr;26(4):951–954. [PubMed] [Google Scholar]
- van der Linden S., Valkenburg H. A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984 Apr;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]


