Abstract
Objectives: To investigate whether anti-Saccharomyces cerevisiae antibodies (ASCA), a marker for Crohn's disease (CD), are present in spondyloarthropathies (SpA) and in the subgroups ankylosing spondylitis (AS), undifferentiated SpA (uSpA), and psoriatic arthritis (PsA), in comparison with healthy and inflammatory controls (patients with rheumatoid arthritis (RA)).
Methods: ASCA IgA and IgG levels were measured with an enzyme linked immunosorbent assay (ELISA) kit (Medipan, Germany) in 26 patients with CD, 108 patients with SpA (43 patients with AS, 20 patients with uSpA, 45 patients with PsA), 56 patients with RA and 45 healthy controls. Gut biopsy samples were available in 18 AS and 10 patients with uSpA, these samples were screened for the presence of inflammation.
Results: Both ASCA IgG and IgA levels were raised in CD compared with healthy controls and patients with RA. ASCA IgA, but not IgG levels, were higher in SpA than in both healthy and RA controls. ASCA IgA levels were raised in AS and uSpA, but not in PsA. No significant differences in ASCA IgA levels were noted between patients with SpA with and without histological gut inflammation.
Conclusion: ASCA IgA levels are significantly higher in SpA, and more specifically in AS, than in healthy controls and patients with RA. This is the first serum marker associated with SpA. No correlation between the presence of subclinical bowel inflammation and ASCA IgA levels was noted. However, it remains to be evaluated whether patients with SpA with ASCA have an increased risk of developing CD.
Full Text
The Full Text of this article is available as a PDF (127.1 KB).
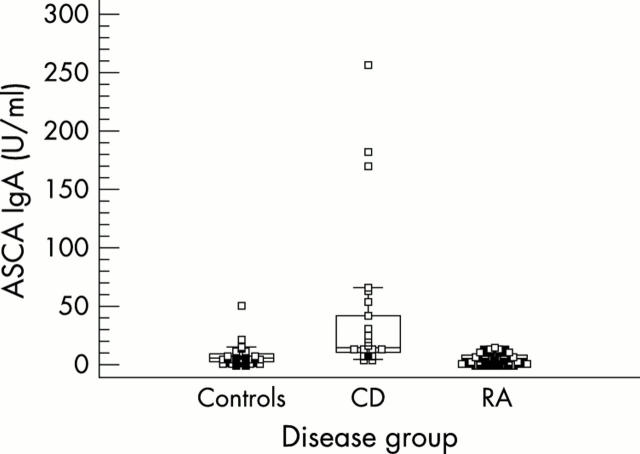
Figure 1.
ASCA IgA levels for healthy controls, patients with CD, and patients with RA. ASCA IgA levels are higher in CD than in controls and patients with RA.
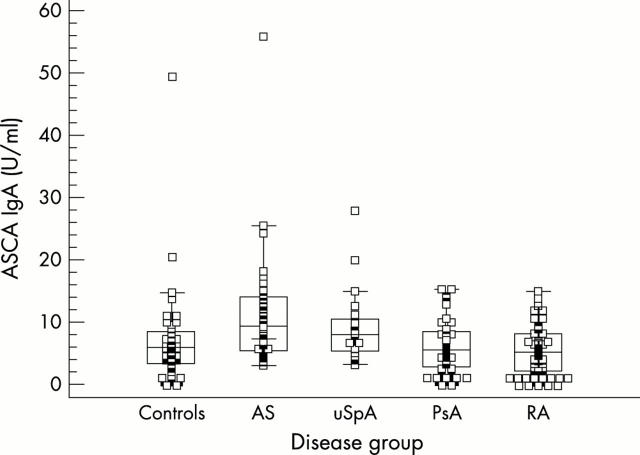
Figure 2.
ASCA IgA levels for healthy controls, patients with AS, uSpA, PsA, and RA. ASCA IgA levels are raised in AS and uSpA, but not in PsA.
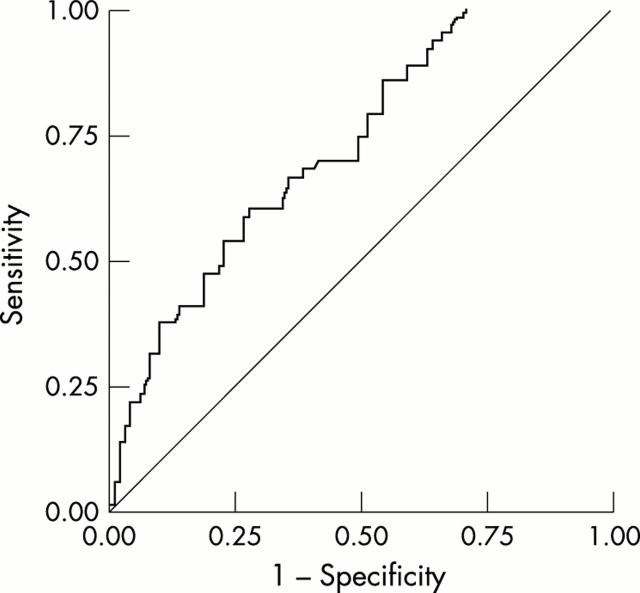
Figure 3.
ROC curve for ASCA IgA, obtained by plotting sensitivity for detecting AS or uSpA (y axis) against specificity (x axis). Both healthy controls and patients with RA were used as the control group for determining specificity. Area under curve = 0.724.
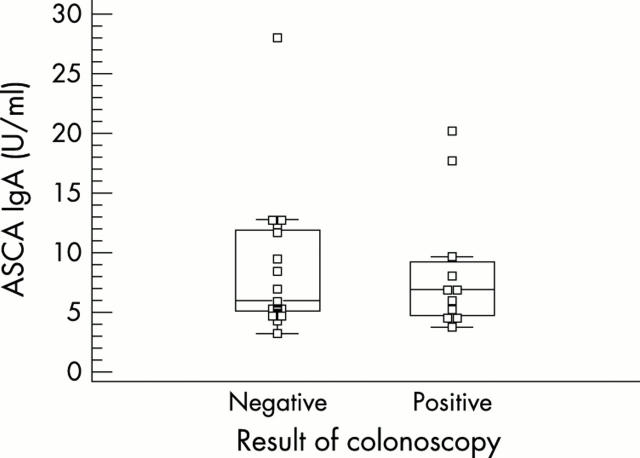
Figure 4.
ASCA IgA levels in patients with SpA with and without bowel inflammation on gut biopsies. No significant difference is noted.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Cuvelier C., Barbatis C., Mielants H., De Vos M., Roels H., Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987 Apr;28(4):394–401. doi: 10.1136/gut.28.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier C., Mielants H., De Vos M., Veys E., Roels H. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut. 1990 May;31(5):545–549. doi: 10.1136/gut.31.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser F., Elewaut D., De Vos M., De Vlam K., Cuvelier C., Mielants H., Veys E. M. Bowel inflammation and the spondyloarthropathies. Rheum Dis Clin North Am. 1998 Nov;24(4):785-813, ix-x. doi: 10.1016/s0889-857x(05)70042-9. [DOI] [PubMed] [Google Scholar]
- De Vos M., Cuvelier C., Mielants H., Veys E., Barbier F., Elewaut A. Ileocolonoscopy in seronegative spondylarthropathy. Gastroenterology. 1989 Feb;96(2 Pt 1):339–344. doi: 10.1016/0016-5085(89)91557-6. [DOI] [PubMed] [Google Scholar]
- Demetter P., Baeten D., De Keyser F., De Vos M., Van Damme N., Verbruggen G., Vermeulen S., Mareel M., Elewaut D., Mielants H. Subclinical gut inflammation in spondyloarthropathy patients is associated with upregulation of the E-cadherin/catenin complex. Ann Rheum Dis. 2000 Mar;59(3):211–216. doi: 10.1136/ard.59.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetter P., De Vos M., Van Damme N., Baeten D., Elewaut D., Vermeulen S., Mareel M., Bullock G., Mielants H., Verbruggen G. Focal up-regulation of E-cadherin-catenin complex in inflamed bowel mucosa but reduced expression in ulcer-associated cell lineage. Am J Clin Pathol. 2000 Sep;114(3):364–370. doi: 10.1093/ajcp/114.3.364. [DOI] [PubMed] [Google Scholar]
- Demetter P., Van Huysse J. A., De Keyser F., Van Damme N., Verbruggen G., Mielants H., De Vos M., Veys E. M., Cuvelier C. A. Increase in lymphoid follicles and leukocyte adhesion molecules emphasizes a role for the gut in spondyloarthropathy pathogenesis. J Pathol. 2002 Dec;198(4):517–522. doi: 10.1002/path.1235. [DOI] [PubMed] [Google Scholar]
- Dougados M., van der Linden S., Juhlin R., Huitfeldt B., Amor B., Calin A., Cats A., Dijkmans B., Olivieri I., Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991 Oct;34(10):1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- Elewaut D., De Keyser F., Cuvelier C., Lazarovits A. I., Mielants H., Verbruggen G., Sas S., Devos M., Veys E. M. Distinctive activated cellular subsets in colon from patients with Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1998 Jul;33(7):743–748. doi: 10.1080/00365529850171693. [DOI] [PubMed] [Google Scholar]
- Hoffenberg E. J., Fidanza S., Sauaia A. Serologic testing for inflammatory bowel disease. J Pediatr. 1999 Apr;134(4):447–452. doi: 10.1016/s0022-3476(99)70202-7. [DOI] [PubMed] [Google Scholar]
- Main J., McKenzie H., Yeaman G. R., Kerr M. A., Robson D., Pennington C. R., Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988 Oct 29;297(6656):1105–1106. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie H., Main J., Pennington C. R., Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990 May;31(5):536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., De Vos M., Goemaere S., De Clercq L., Schatteman L., Elewaut D. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J Rheumatol. 1995 Dec;22(12):2273–2278. [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., De Vos M., Goemaere S., De Clercq L., Schatteman L., Gyselbrecht L., Elewaut D. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol. 1995 Dec;22(12):2279–2284. [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27 (Suppl 2):95–105. doi: 10.1093/rheumatology/xxvii.suppl_2.95. [DOI] [PubMed] [Google Scholar]
- Peeters M., Joossens S., Vermeire S., Vlietinck R., Bossuyt X., Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001 Mar;96(3):730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- Quinton J. F., Sendid B., Reumaux D., Duthilleul P., Cortot A., Grandbastien B., Charrier G., Targan S. R., Colombel J. F., Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998 Jun;42(6):788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruemmele F. M., Targan S. R., Levy G., Dubinsky M., Braun J., Seidman E. G. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998 Oct;115(4):822–829. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- Schatteman L., Mielants H., Veys E. M., Cuvelier C., De Vos M., Gyselbrecht L., Elewaut D., Goemaere S. Gut inflammation in psoriatic arthritis: a prospective ileocolonoscopic study. J Rheumatol. 1995 Apr;22(4):680–683. [PubMed] [Google Scholar]
- Sendid B., Colombel J. F., Jacquinot P. M., Faille C., Fruit J., Cortot A., Lucidarme D., Camus D., Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996 Mar;3(2):219–226. doi: 10.1128/cdli.3.2.219-226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendid B., Quinton J. F., Charrier G., Goulet O., Cortot A., Grandbastien B., Poulain D., Colombel J. F. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn's disease. Am J Gastroenterol. 1998 Aug;93(8):1306–1310. doi: 10.1111/j.1572-0241.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- Targan S. R., Hanauer S. B., van Deventer S. J., Mayer L., Present D. H., Braakman T., DeWoody K. L., Schaible T. F., Rutgeerts P. J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997 Oct 9;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Van Damme N., De Keyser F., Demetter P., Baeten D., Mielants H., Verbruggen G., Cuvelier C., Veys E. M., De Vos M. The proportion of Th1 cells, which prevail in gut mucosa, is decreased in inflammatory bowel syndrome. Clin Exp Immunol. 2001 Sep;125(3):383–390. doi: 10.1046/j.1365-2249.2001.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N., De Vos M., Baeten D., Demetter P., Mielants H., Verbruggen G., Cuvelier C., Veys E. M., De Keyser F. Flow cytometric analysis of gut mucosal lymphocytes supports an impaired Th1 cytokine profile in spondyloarthropathy. Ann Rheum Dis. 2001 May;60(5):495–499. doi: 10.1136/ard.60.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N., Elewaut D., Baeten D., Demetter P., Cuvelier C., Verbruggen G., Mielants H., Veys E. M., De Vos M., De Keyser F. Gut mucosal T cell lines from ankylosing spondylitis patients are enriched with alphaEbeta7 integrin. Clin Exp Rheumatol. 2001 Nov-Dec;19(6):681–687. [PubMed] [Google Scholar]
- Van Den Bosch Filip, Kruithof Elli, Baeten Dominique, Herssens Annemie, de Keyser Filip, Mielants Herman, Veys Eric M. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum. 2002 Mar;46(3):755–765. doi: 10.1002/art.511. [DOI] [PubMed] [Google Scholar]
- Van den Bosch F., Kruithof E., Baeten D., De Keyser F., Mielants H., Veys E. M. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis. 2000 Jun;59(6):428–433. doi: 10.1136/ard.59.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bosch F., Kruithof E., De Vos M., De Keyser F., Mielants H. Crohn's disease associated with spondyloarthropathy: effect of TNF-alpha blockade with infliximab on articular symptoms. Lancet. 2000 Nov 25;356(9244):1821–1822. doi: 10.1016/s0140-6736(00)03239-6. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas E. A., Kam L. Y., Karp L. C., Gaiennie J., Yang H., Targan S. R. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000 Oct;47(4):487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S., Joossens S., Peeters M., Monsuur F., Marien G., Bossuyt X., Groenen P., Vlietinck R., Rutgeerts P. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology. 2001 Mar;120(4):827–833. doi: 10.1053/gast.2001.22546. [DOI] [PubMed] [Google Scholar]
- Vermeire S., Peeters M., Vlietinck R., Joossens S., Den Hond E., Bulteel V., Bossuyt X., Geypens B., Rutgeerts P. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis. 2001 Feb;7(1):8–15. doi: 10.1097/00054725-200102000-00002. [DOI] [PubMed] [Google Scholar]
- de Vlam K., Mielants H., Cuvelier C., De Keyser F., Veys E. M., De Vos M. Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol. 2000 Dec;27(12):2860–2865. [PubMed] [Google Scholar]
- van der Linden S., Valkenburg H. A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984 Apr;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]