Abstract
Objective: To evaluate whether, in patients with the diffuse form of systemic sclerosis (dSSc), macrophage migration inhibitory factor (MIF) production is dysregulated.
Methods: 10 patients with dSSc and 10 healthy controls, matched for age and sex, were studied. MIF expression was evaluated by immunohistochemistry on formalin fixed skin biopsies of patients with dSSc and controls. MIF levels were assayed in the sera and in the supernatants of skin cultured fibroblasts by a colorimetric sandwich enzyme linked immunosorbent assay (ELISA). MIF concentrations in culture medium samples and in serum samples were compared by Student's two tailed t test for unpaired data.
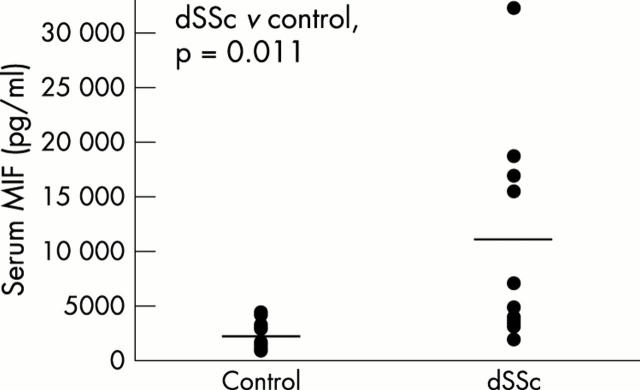
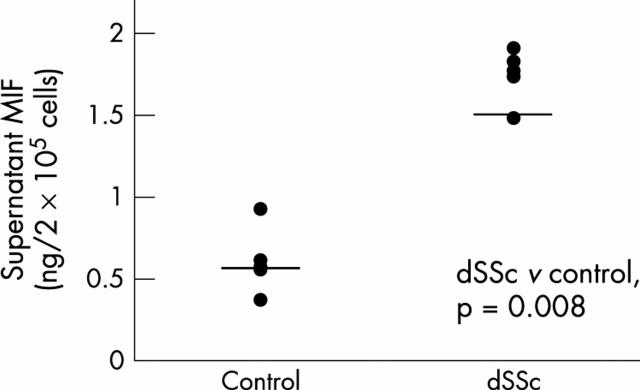
Results: Anti-MIF antibody immunostained the basal and mainly suprabasal keratinocytes. Small perivascular clusters of infiltrating mononuclear cells were positive; scattered spindle fibroblast-like cells were immunostained in superficial and deep dermal layers. The serum concentrations of MIF in patients with dSSc (mean (SD) 10705.6 (9311) pg/ml) were significantly higher than in controls (2157.5 (1288.6) pg/ml; p=0.011); MIF levels from dSSc fibroblast cultures (mean (SD) 1.74 (0.16) ng/2x105 cells) were also significantly higher than in controls (0.6 (0.2) ng/2x105 cells; p=0.008).
Conclusion: These results suggest that MIF may be involved in the amplifying proinflammatory loop leading to scleroderma tissue remodelling.
Full Text
The Full Text of this article is available as a PDF (151.0 KB).
Figure 1.
Serum MIF levels (pg/ml) from 10 patients with dSSc and 10 healthy controls matched for age and sex. The concentration of MIF in patients with dSSc was significantly higher than in controls (p<0.05; Mann-Whitney rank sum test).
Figure 2.
Supernatant MIF levels from fibroblast cell cultures (ng/2x105cell). The samples were obtained from five patients with dSSc and five healthy controls matched for age and sex. The concentration of MIF in patients with dSSc was significantly higher than in controls (p<0.01; Mann-Whitney rank sum test).
Figure 3.
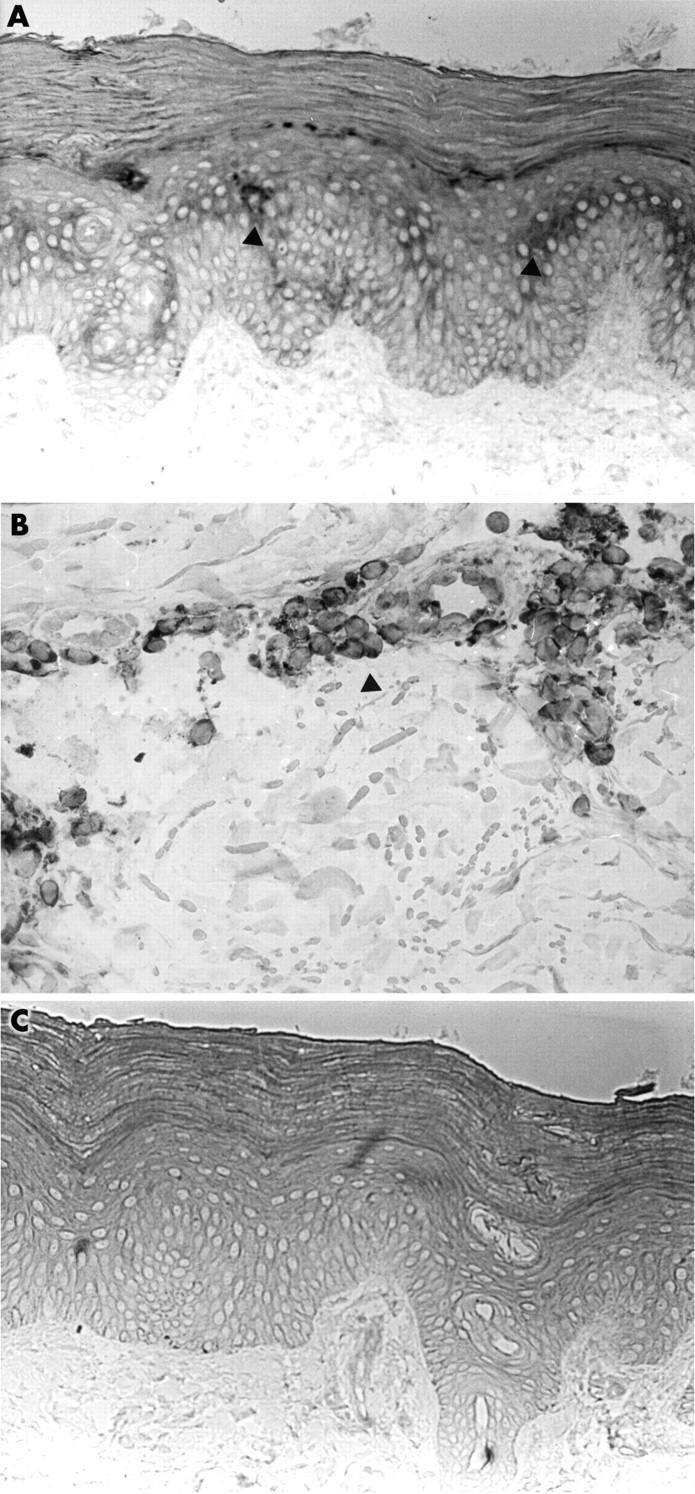
(A, B) Sclerodermal skin section immunostained by anti-MIF antibody. (A) Positive basal and suprabasal keratinocytes (arrow head). Original magnification x20. (B) Several positive perivascular mononuclear cells (arrow head). Original magnification x60. Antigen retrieval was carried out by incubating sections with the antihuman MIF goat polyclonal antibody diluted 1:300 in TBS. The reaction was shown using the streptavidin-biotin complex. (C) Negative control: SSc skin section immunostained by replacing the specific antibody with non-immune serum immunoglobulins at the same concentration as the primary antibody. Original magnification x20.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Shimizu T., Ohkawara A., Nishihira J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim Biophys Acta. 2000 Jan 3;1500(1):1–9. doi: 10.1016/s0925-4439(99)00080-0. [DOI] [PubMed] [Google Scholar]
- Arcuri F., Ricci C., Ietta F., Cintorino M., Tripodi S. A., Cetin I., Garzia E., Schatz F., Klemi P., Santopietro R. Macrophage migration inhibitory factor in the human endometrium: expression and localization during the menstrual cycle and early pregnancy. Biol Reprod. 2001 Apr;64(4):1200–1205. doi: 10.1095/biolreprod64.4.1200. [DOI] [PubMed] [Google Scholar]
- Attur M. G., Patel R. N., Abramson S. B., Amin A. R. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 1997 Jun;40(6):1050–1053. doi: 10.1002/art.1780400609. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Calandra T., Mitchell R. A., Martin S. B., Tracey K. J., Voelter W., Manogue K. R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993 Oct 21;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995 Sep 7;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Cunha F. Q., Weiser W. Y., David J. R., Moss D. W., Moncada S., Liew F. Y. Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages. J Immunol. 1993 Mar 1;150(5):1908–1912. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali C. A., Bost K. L., Boulware D. W., Levy L. S. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992 Aug;19(8):1207–1211. [PubMed] [Google Scholar]
- Galindo M., Santiago B., Rivero M., Rullas J., Alcami J., Pablos J. L. Chemokine expression by systemic sclerosis fibroblasts: abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 2001 Jun;44(6):1382–1386. doi: 10.1002/1529-0131(200106)44:6<1382::AID-ART231>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kahaleh M. B., Sultany G. L., Smith E. A., Huffstutter J. E., Loadholt C. B., LeRoy E. C. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986 Oct-Dec;4(4):367–369. [PubMed] [Google Scholar]
- Kawaguchi Y., Harigai M., Hara M., Suzuki K., Kawakami M., Ishizuka T., Hidaka T., Kitani A., Kawagoe M., Nakamura H. Increased interleukin 1 receptor, type I, at messenger RNA and protein level in skin fibroblasts from patients with systemic sclerosis. Biochem Biophys Res Commun. 1992 May 15;184(3):1504–1510. doi: 10.1016/s0006-291x(05)80053-1. [DOI] [PubMed] [Google Scholar]
- Kondo H., Rabin B. S., Rodnan G. P. Cutaneous antigen-stimulating lymphokine production by lymphocytes of patients with progressive systemic sclerosis (scleroderma). J Clin Invest. 1976 Dec;58(6):1388–1394. doi: 10.1172/JCI108594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Rabin B. S., Rodnan G. P. Stimulation of lymphocyte reactivity by a low molecular weight cutaneous antigen in patients with progressive systemic sclerosis (scleroderma). J Rheumatol. 1979 Jan-Feb;6(1):30–37. [PubMed] [Google Scholar]
- LeRoy E. C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T. A., Jr, Rowell N., Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–205. [PubMed] [Google Scholar]
- Leech M., Metz C., Hall P., Hutchinson P., Gianis K., Smith M., Weedon H., Holdsworth S. R., Bucala R., Morand E. F. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999 Aug;42(8):1601–1608. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Leech M., Metz C., Santos L., Peng T., Holdsworth S. R., Bucala R., Morand E. F. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 1998 May;41(5):910–917. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Regulation of nitric oxide synthesis in infectious and autoimmune diseases. Immunol Lett. 1994 Dec;43(1-2):95–98. doi: 10.1016/0165-2478(94)00157-x. [DOI] [PubMed] [Google Scholar]
- Lolis E. Glucocorticoid counter regulation: macrophage migration inhibitory factor as a target for drug discovery. Curr Opin Pharmacol. 2001 Dec;1(6):662–668. doi: 10.1016/s1471-4892(01)00112-6. [DOI] [PubMed] [Google Scholar]
- Matsuda A., Tagawa Y., Matsuda H., Nishihira J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996 May 6;385(3):225–228. doi: 10.1016/0014-5793(96)00386-9. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., Leung B. P., Field M., Wei X. Q., Huang F. P., Sturrock R. D., Kinninmonth A., Weidner J., Mumford R., Liew F. Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996 Oct 1;184(4):1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meazza Cristina, Travaglino Paola, Pignatti Patrizia, Magni-Manzoni Silvia, Ravelli Angelo, Martini Alberto, De Benedetti Fabrizio. Macrophage migration inhibitory factor in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002 Jan;46(1):232–237. doi: 10.1002/1529-0131(200201)46:1<232::AID-ART10059>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera S., Suzuki K., Matsuno T., Kaneda K., Kuriyama T., Nishihira J. Identification of macrophage migration inhibitory factor in murine neonatal calvariae and osteoblasts. Immunology. 1996 Nov;89(3):430–435. doi: 10.1046/j.1365-2567.1996.d01-751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Sampey A. V., Hall P. H., Mitchell R. A., Metz C. N., Morand E. F. Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis Rheum. 2001 Jun;44(6):1273–1280. doi: 10.1002/1529-0131(200106)44:6<1273::AID-ART219>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Abe R., Ohkawara A., Mizue Y., Nishihira J. Macrophage migration inhibitory factor is an essential immunoregulatory cytokine in atopic dermatitis. Biochem Biophys Res Commun. 1997 Nov 7;240(1):173–178. doi: 10.1006/bbrc.1997.7633. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohkawara A., Mizue Y., Nishihira J. Alpha-thrombin stimulates expression of macrophage migration inhibitory factor in skin fibroblasts. Semin Thromb Hemost. 1999;25(6):569–573. doi: 10.1055/s-2007-994967. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ohkawara A., Nishihira J., Sakamoto W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immmunohistochemical localization. FEBS Lett. 1996 Mar 4;381(3):199–202. doi: 10.1016/0014-5793(96)00120-2. [DOI] [PubMed] [Google Scholar]
- Steinhoff M., Meinhardt A., Steinhoff A., Gemsa D., Bucala R., Bacher M. Evidence for a role of macrophage migration inhibitory factor in psoriatic skin disease. Br J Dermatol. 1999 Dec;141(6):1061–1066. doi: 10.1046/j.1365-2133.1999.03206.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Nishihira J., Sato Y., Kondo M., Ogawa H., Ohshima T., Une Y., Todo S. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med. 1998 Nov;4(11):707–714. [PMC free article] [PubMed] [Google Scholar]
- Yamakage A., Kikuchi K., Smith E. A., LeRoy E. C., Trojanowska M. Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J Exp Med. 1992 May 1;175(5):1227–1234. doi: 10.1084/jem.175.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]