Abstract
Background: Systemic lupus erythematosus (SLE) is characterised by the presence of antibodies to double stranded DNA (dsDNA), which are involved in the pathogenesis of SLE. Previous studies showed that at least two thirds of patients develop a clinical relapse within six months after a significant rise in the anti-dsDNA level, and most relapses were prevented by the administration of corticosteroids at the time of the rise.
Objective: To determine whether mofetil mycophenolate (MMF) can prevent a clinical relapse without the side effects associated with corticosteroids.
Methods: 36 patients with SLE were examined monthly to determine whether a rise in anti-dsDNA level had occurred. A rise was defined as an increase of 25% of the level of the previous sample of at least 15 IU/ml within a four month period. After a rise patients were treated with MMF 2000 mg daily for six months. Patients were monitored monthly for the occurrence of a clinical relapse and to assess the serological activity and state of activation of CD4+, CD8+, and CD19+ lymphocyte subsets.
Results: Anti-dsDNA rose in 10 patients. Treatment with MMF was started in all these patients, and after six months no clinical relapse had occurred. Side effects were minimal. Antibodies to dsDNA decreased during the treatment (p<0.001), associated with a decrease in the state of activation of CD19+ lymphocytes. No changes were found in the state of activation of CD4+ or CD8+ lymphocyte subsets.
Conclusion: Administration of MMF after a rise in antibodies to dsDNA is well tolerated, decreases anti-dsDNA and B cell activation, and seems to prevent the occurrence of a clinical relapse in patients with SLE.
Full Text
The Full Text of this article is available as a PDF (157.3 KB).
Figure 1.
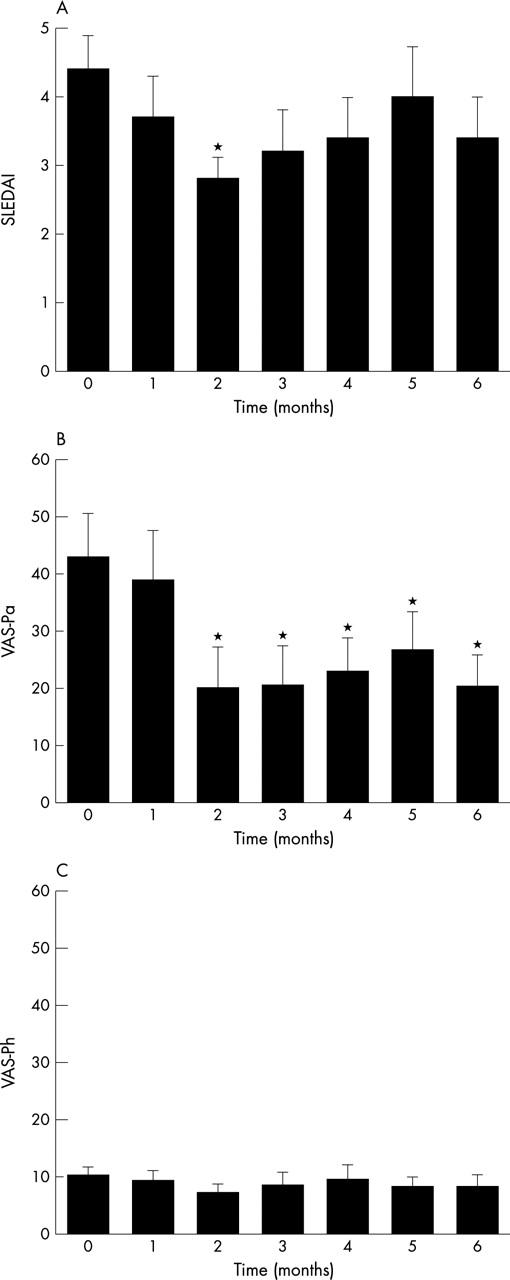
(A) SLEDAI score of 10 patients during a six month period of treatment with MMF after a significant rise in the level of antibodies to dsDNA. (B) VAS-Pa and (C) VAS-Ph represent the impression of the patient and the treating physician, respectively, of disease activity during the month before the visit to the outpatient clinic. *p<0.05, comparing different time points of evaluation with that at the start of treatment at time 0 (repeated measures analysis of variance with Bonferroni's post-test). Data are presented as mean (SE).
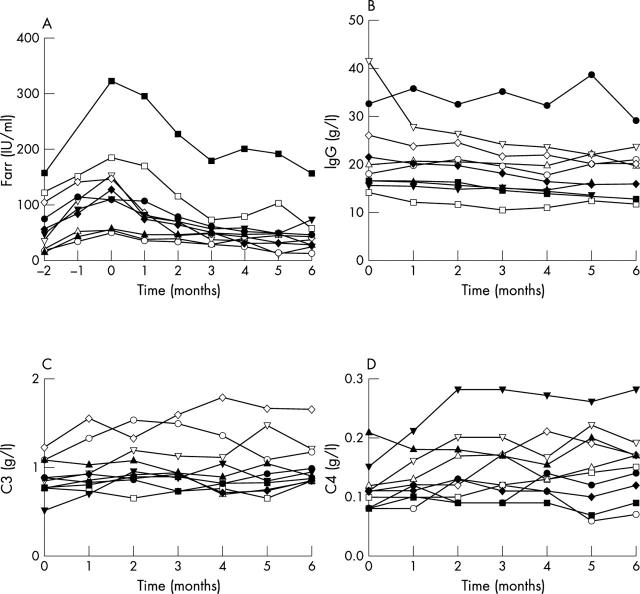
Figure 2.
Levels of antibodies to dsDNA of each patient are shown from two months before the significant rise was established at t=0, until six months thereafter. MMF was started at t=0 and continued for six months (A). For each participant, levels of total IgG (B), complement C3 (C), and complement C4 (D) are also shown. In the graphs each individual patient is represented by a different symbol.
Figure 3.
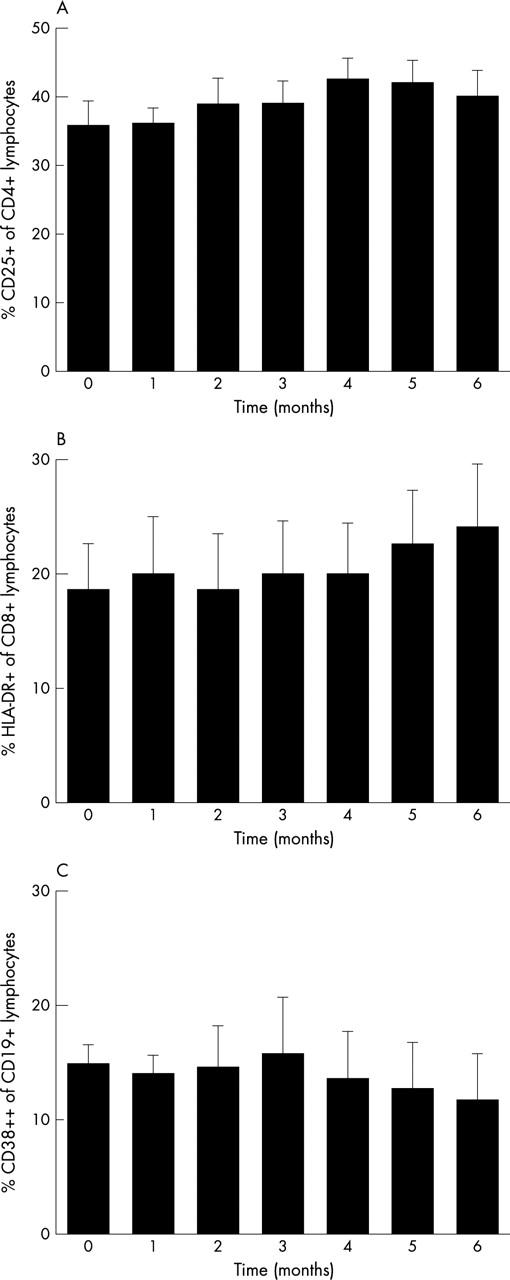
Activation state of lymphocytes during six months of treatment with MMF. (A) Activation state of CD4+ lymphocytes depicted as the percentage of these cells staining positive for the activation marker CD25. (B) Activation state of CD8+ lymphocytes depicted as the percentage staining positive for HLA-DR. (C) Activation state of CD19+ lymphocytes expressed as the percentage of CD38++ lymphocytes. Data are presented as mean (SE).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuckle M. R., James J. A., Kohlhase K. F., Rubertone M. V., Dennis G. J., Harley J. B. Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand J Immunol. 2001 Jul-Aug;54(1-2):211–219. doi: 10.1046/j.1365-3083.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- Bijl M., van Lopik T., Limburg P. C., Spronk P. E., Jaegers S. M., Aarden L. A., Smeenk R. J., Kallenberg G. G. Do elevated levels of serum-soluble fas contribute to the persistence of activated lymphocytes in systemic lupus erythematosus? J Autoimmun. 1998 Oct;11(5):457–463. doi: 10.1006/jaut.1998.0233. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Bootsma H., Spronk P., Derksen R., de Boer G., Wolters-Dicke H., Hermans J., Limburg P., Gmelig-Meyling F., Kater L., Kallenberg C. Prevention of relapses in systemic lupus erythematosus. Lancet. 1995 Jun 24;345(8965):1595–1599. doi: 10.1016/s0140-6736(95)90114-0. [DOI] [PubMed] [Google Scholar]
- Chan T. M., Li F. K., Tang C. S., Wong R. W., Fang G. X., Ji Y. L., Lau C. S., Wong A. K., Tong M. K., Chan K. W. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000 Oct 19;343(16):1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- Esdaile J. M., Abrahamowicz M., Joseph L., MacKenzie T., Li Y., Danoff D. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996 Mar;39(3):370–378. doi: 10.1002/art.1780390304. [DOI] [PubMed] [Google Scholar]
- Feltkamp T. E., Kirkwood T. B., Maini R. N., Aarden L. A. The first international standard for antibodies to double stranded DNA. Ann Rheum Dis. 1988 Sep;47(9):740–746. doi: 10.1136/ard.47.9.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B., Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996 Feb;51(2):278–298. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- Gaubitz M., Schorat A., Schotte H., Kern P., Domschke W. Mycophenolate mofetil for the treatment of systemic lupus erythematosus: an open pilot trial. Lupus. 1999;8(9):731–736. doi: 10.1191/096120399678840927. [DOI] [PubMed] [Google Scholar]
- Glicklich D., Acharya A. Mycophenolate mofetil therapy for lupus nephritis refractory to intravenous cyclophosphamide. Am J Kidney Dis. 1998 Aug;32(2):318–322. doi: 10.1053/ajkd.1998.v32.pm9708620. [DOI] [PubMed] [Google Scholar]
- Hahn B. H. Antibodies to DNA. N Engl J Med. 1998 May 7;338(19):1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- Heinschink A., Raab M., Daxecker H., Griesmacher A., Müller M. M. In vitro effects of mycophenolic acid on cell cycle and activation of human lymphocytes. Clin Chim Acta. 2000 Oct;300(1-2):23–28. doi: 10.1016/s0009-8981(00)00297-7. [DOI] [PubMed] [Google Scholar]
- Ho A., Magder L. S., Barr S. G., Petri M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001 Oct;44(10):2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Petri M., Genovese M., Engle E., Hochberg M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991 Aug;34(8):937–944. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- Spronk P. E., Horst G., Van Der Gun B. T., Limburg P. C., Kallenberg C. G. Anti-dsDNA production coincides with concurrent B and T cell activation during development of active disease in systemic lupus erythematosus (SLE). Clin Exp Immunol. 1996 Jun;104(3):446–453. doi: 10.1046/j.1365-2249.1996.44754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk P. E., vd Gun B. T., Limburg P. C., Kallenberg C. G. B cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to levels of anti-dsDNA, nor to concurrent T cell activation. Clin Exp Immunol. 1993 Jul;93(1):39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaak A. J., Groenwold J., Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis. 1986 May;45(5):359–366. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Walz LeBlanc B. A., Gladman D. D., Urowitz M. B. Serologically active clinically quiescent systemic lupus erythematosus--predictors of clinical flares. J Rheumatol. 1994 Dec;21(12):2239–2241. [PubMed] [Google Scholar]
- Zonana-Nacach A., Barr S. G., Magder L. S., Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000 Aug;43(8):1801–1808. doi: 10.1002/1529-0131(200008)43:8<1801::AID-ANR16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E. J., Limburg P. C., Kallenberg C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990 May;33(5):634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]