Abstract
Background: Recently it has been suggested that microchimerism may have a significant role in the aetiopathogenesis of some autoimmune diseases.
Objectives: To evaluate the incidence of microchimerism in systemic lupus erythematosus (SLE), to quantify the phenomenon, to evaluate changes of microchimerism during follow up, and to correlate these data with clinical and laboratory variables.
Methods: Patients were selected for the study on the basis of the following criteria: (a) pregnancy with at least one male offspring; (b) no history of abortion and blood transfusion. Microchimerism was detected using a competitive nested polymerase chain reaction for a specific Y chromosome sequence and an internal competitor designed ad hoc. Disease activity and organ involvement were also evaluated.
Results: Sixty samples from 22 patients with SLE and 24 healthy controls were examined. Microchimerism was seen in 11 (50%) patients and 12 (50%) controls. The mean number of male equivalent cells was 2.4 cells/100 000 (range 0.1–17) in patients with SLE and 2.5 (range 0.2–1.8) in healthy controls. No differences in the incidence of microchimerism or in the number of microchimeric cells were found between patients and healthy controls. Patients with a history of lupus nephritis had a higher mean number of fetal cells than patients with no such history. Disease activity did not appear to correlate with microchimerism.
Conclusions: The preliminary data suggest that microchimerism does not interfere with the disease course of SLE, although further analysis on larger groups will be necessary to confirm these observations.
Full Text
The Full Text of this article is available as a PDF (97.3 KB).
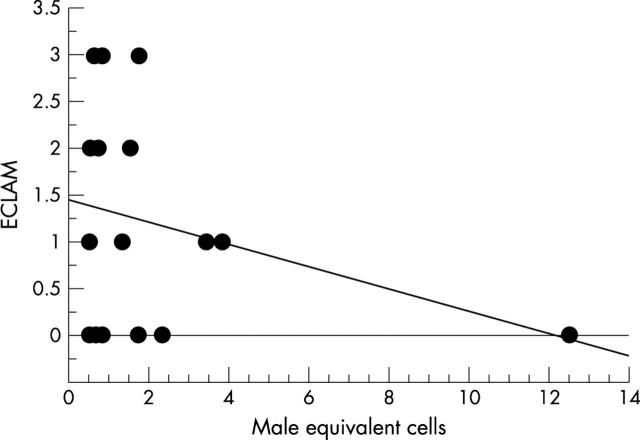
Figure 1.
Correlation between the number of chimeric cells and the ECLAM index in patients with SLE.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artlett C. M., Cox L. A., Jimenez S. A. Detection of cellular microchimerism of male or female origin in systemic sclerosis patients by polymerase chain reaction analysis of HLA-Cw antigens. Arthritis Rheum. 2000 May;43(5):1062–1067. doi: 10.1002/1529-0131(200005)43:5<1062::AID-ANR16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Cox L. A., Jimenez S. A. Detection of cellular microchimerism of male or female origin in systemic sclerosis patients by polymerase chain reaction analysis of HLA-Cw antigens. Arthritis Rheum. 2000 May;43(5):1062–1067. doi: 10.1002/1529-0131(200005)43:5<1062::AID-ANR16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Miller F. W., Rider L. G., Childhood Myositis Heterogeneity Collaborative Study Group Persistent maternally derived peripheral microchimerism is associated with the juvenile idiopathic inflammatory myopathies. Rheumatology (Oxford) 2001 Nov;40(11):1279–1284. doi: 10.1093/rheumatology/40.11.1279. [DOI] [PubMed] [Google Scholar]
- Artlett C. M., Smith J. B., Jimenez S. A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998 Apr 23;338(17):1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- Bianchi D. W., Zickwolf G. K., Weil G. J., Sylvester S., DeMaria M. A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci F., Priori R., Alessandri C., Valesini G., Stoppacciaro A. Y chromosome microchimerism in Sjögren's syndrome. Ann Rheum Dis. 2001 Nov;60(11):1078–1079. doi: 10.1136/ard.60.11.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. C., Lambert N., Maloney S., Furst D. E., Moore J. M., Nelson J. L. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999 Mar 15;93(6):2033–2037. [PubMed] [Google Scholar]
- Hochberg M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725–1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Ichikawa N., Kotake S., Hakoda M., Kamatani N. Microchimerism in Japanese patients with systemic sclerosis. Arthritis Rheum. 2001 May;44(5):1226–1228. doi: 10.1002/1529-0131(200105)44:5<1226::AID-ANR208>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Invernizzi P., De Andreis C., Sirchia S. M., Battezzati P. M., Zuin M., Rossella F., Perego F., Bignotto M., Simoni G., Podda M. Blood fetal microchimerism in primary biliary cirrhosis. Clin Exp Immunol. 2000 Dec;122(3):418–422. doi: 10.1046/j.1365-2249.2000.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. L., McAlindon T. E., Mulcahy E., Bianchi D. W. Microchimerism in a female patient with systemic lupus erythematosus. Arthritis Rheum. 2001 Sep;44(9):2107–2111. doi: 10.1002/1529-0131(200109)44:9<2107::AID-ART361>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lambert N. C., Evans P. C., Hashizumi T. L., Maloney S., Gooley T., Furst D. E., Nelson J. L. Cutting edge: persistent fetal microchimerism in T lymphocytes is associated with HLA-DQA1*0501: implications in autoimmunity. J Immunol. 2000 Jun 1;164(11):5545–5548. doi: 10.4049/jimmunol.164.11.5545. [DOI] [PubMed] [Google Scholar]
- Lambert N. C., Stevens A. M., Tylee T. S., Erickson T. D., Furst D. E., Nelson J. L. From the simple detection of microchimerism in patients with autoimmune diseases to its implication in pathogenesis. Ann N Y Acad Sci. 2001 Sep;945:164–171. doi: 10.1111/j.1749-6632.2001.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Paglieroni T., Ohto H., Holland P. V., Busch M. P. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999 May 1;93(9):3127–3139. [PubMed] [Google Scholar]
- Murata H., Nakauchi H., Sumida T. Microchimerism in Japanese women patients with systemic sclerosis. Lancet. 1999 Jul 17;354(9174):220–220. doi: 10.1016/S0140-6736(99)00164-6. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Furst D. E., Maloney S., Gooley T., Evans P. C., Smith A., Bean M. A., Ober C., Bianchi D. W. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998 Feb 21;351(9102):559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Nelson J. L. HLA relationships of pregnancy, microchimerism and autoimmune disease. J Reprod Immunol. 2001 Oct-Nov;52(1-2):77–84. doi: 10.1016/s0165-0378(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Nelson J. L. Microchimerism: implications for autoimmune disease. Lupus. 1999;8(5):370–374. doi: 10.1177/096120339900800508. [DOI] [PubMed] [Google Scholar]
- Nelson J. Lee. Microchimerism: incidental byproduct of pregnancy or active participant in human health? Trends Mol Med. 2002 Mar;8(3):109–113. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Luk K. C., Williams B., Lifson J. D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. Biotechniques. 1993 Jan;14(1):70–81. [PubMed] [Google Scholar]
- Reed A. M., Picornell Y. J., Harwood A., Kredich D. W. Chimerism in children with juvenile dermatomyositis. Lancet. 2000 Dec 23;356(9248):2156–2157. doi: 10.1016/S0140-6736(00)03500-5. [DOI] [PubMed] [Google Scholar]
- Scaletti Cristina, Vultaggio Alessandra, Bonifacio Stefania, Emmi Lorenzo, Torricelli Francesca, Maggi Enrico, Romagnani Sergio, Piccinni Marie-Pierre. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002 Feb;46(2):445–450. doi: 10.1002/art.10049. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques. 1993 Feb;14(2):244–249. [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Trucco M., Murase N., Ricordi C., Ildstad S., Ramos H., Todo S., Tzakis A., Fung J. J. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993 Jun;17(6):1127–1152. [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Murase N. Microchimerism, macrochimerism, and tolerance. Clin Transplant. 2000 Aug;14(4 Pt 1):351–354. doi: 10.1034/j.1399-0012.2000.t01-1-140412.x. [DOI] [PubMed] [Google Scholar]
- Toda I., Kuwana M., Tsubota K., Kawakami Y. Lack of evidence for an increased microchimerism in the circulation of patients with Sjögren's syndrome. Ann Rheum Dis. 2001 Mar;60(3):248–253. doi: 10.1136/ard.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C., Bencivelli W., Isenberg D. A., Smolen J. S., Snaith M. L., Sciuto M., Neri R., Bombardieri S. Disease activity in systemic lupus erythematosus: report of the Consensus Study Group of the European Workshop for Rheumatology Research. II. Identification of the variables indicative of disease activity and their use in the development of an activity score. The European Consensus Study Group for Disease Activity in SLE. Clin Exp Rheumatol. 1992 Sep-Oct;10(5):541–547. [PubMed] [Google Scholar]