Abstract
Background: Therapeutic strategies to block tumour necrosis factor α (TNFα) activity in experimental autoimmune arthritis models and rheumatoid arthritis (RA) have proved highly successful, and provide sustained beneficial effects.
Objective: To examine whether TNFα inhibition has immunological activity beyond the reduction of inflammation in collagen induced arthritis (CIA), an established experimental model of RA.
Methods: Arthritic DBA/1 mice received single periarticular injections of retroviral constructs encoding human TNF receptor (TNF-R) into the affected arthritic paw, at the onset of arthritis. Severity of arthritis, antibodies to collagen type II (CII), and extent of pathological joint damage of arthritic paws were compared between TNF-R and media treated (control) animals 3, 7, 14, 21, and 49 days after disease onset.
Results: Severity of CIA was significantly decreased in TNF-R treated animals compared with controls, 14–34 days after disease onset. Joint destruction was reduced in TNF-R injected joints and in the uninjected contralateral and ipsilateral paws of TNF-R treated animals. Seven days after disease onset, TNF-R treated mice had lower levels of inflammatory Th1 driven IgG2a antibodies to CII (p<0.05) than controls. This altered the anticollagen IgG2a:IgG1 ratio towards Th2 driven IgG1.
Conclusions: Local TNF-R gene therapy in CIA appears to have systemic effects on the anti-CII antibodies. The overall influence of TNF-R gene therapy is that it inhibits the progression of CIA mainly by suppressing the inflammatory Th1 response rather than by stimulating a Th2 response. Therefore, periarticular TNF-R gene therapy may have excellent therapeutic potential in RA.
Full Text
The Full Text of this article is available as a PDF (346.0 KB).
Figure 1.
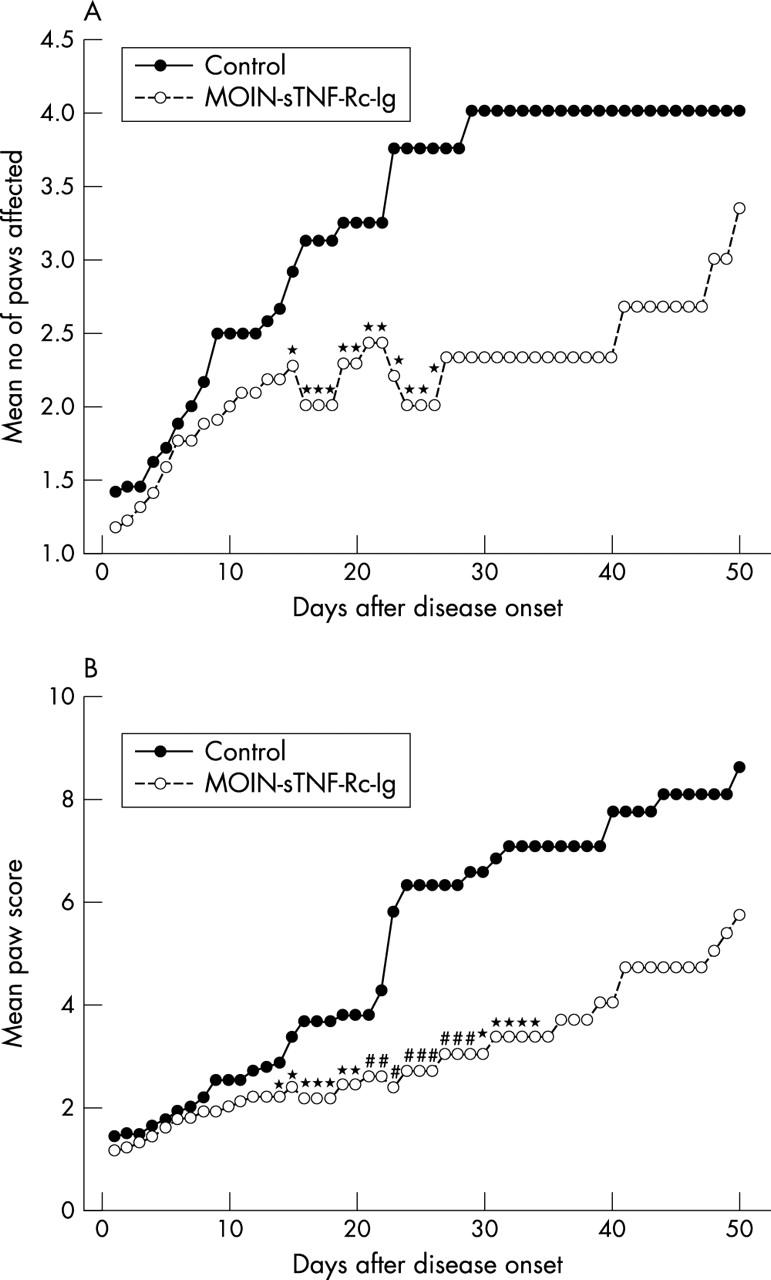
Effect of a single periarticular delivery of retrovirus mediated MOIN-sTNF-Rc-Ig on the clinical severity of CIA. At arthritis onset 100 µl of media alone (n=24) or 1.6x107 pfu/ml of MOIN-sTNF-Rc-Ig in 100 µl of media (n=22) was injected periarticularly into the affected arthritic paw. Disease activity represented by (A) the mean number of paws affected and (B) the mean paw score was visually scored up to 49 days after disease onset. Data are expressed as mean for each group. SEM <5% of mean (not shown). Statistically significant differences between the two groups is indicated: *p<0.05; #p<0.02). The figure shows representative results from two different individual experiments, which gave similar results.
Figure 2.
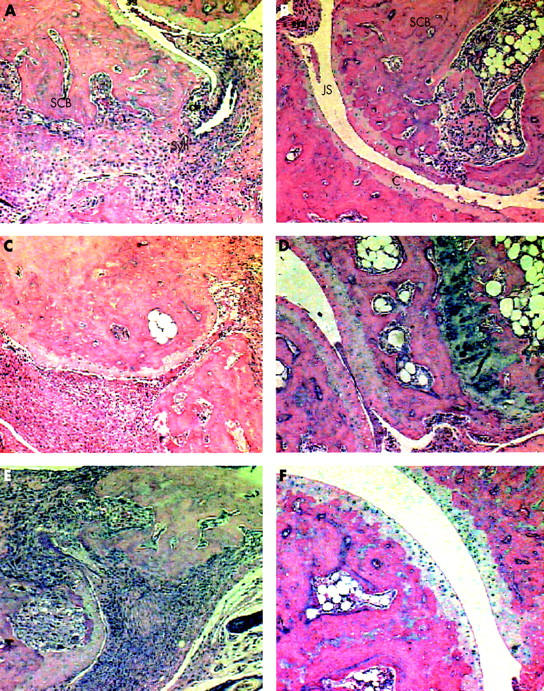
Histopathology of injected, contralateral, and ipsilateral joints. Histological evaluation of control injected (A), contralateral (C), and ipsilateral (E) and MOIN-sTNF-Rc-Ig injected (B), contralateral (D), and ipsilateral (F) arthritic joints were conducted at 49 days after disease onset and at the start of treatment. Media injected control joints show severe synovial inflammation with clear pannus attachment and erosions extending into the subchondral bone, indicating destructive erosive disease. Similar pathological changes are seen in the control arthritic contralateral and ipsilateral joints. Injected, contralateral, and ipsilateral arthritic joints of MOIN-sTNF-Rc-Ig treated mice show a lesser degree of both synovial inflammation and destructive disease, indicating a relatively normal joint architecture. Haematoxylin and eosin staining (magnification x100) used for all sections. JS, joint space; SCB, subchondral bone; CPJ, cartilage/pannus junction; Syn, synovium; P, pannus.
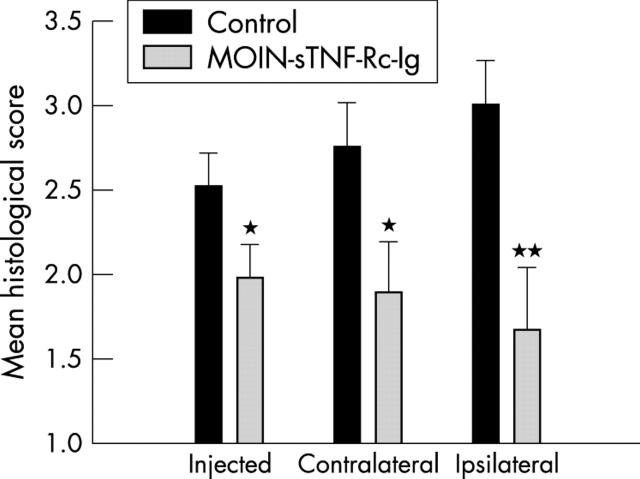
Figure 3.
Histological scoring of arthritic joints. Arthritic joints were analysed by histology using haematoxylin and eosin staining for extent of joint damage and assigned a histological score (as described in "Materials and methods"). Each column represents the mean scores (SEM) for the total number of mice in each group. *p < 0.05; **p<0.01.
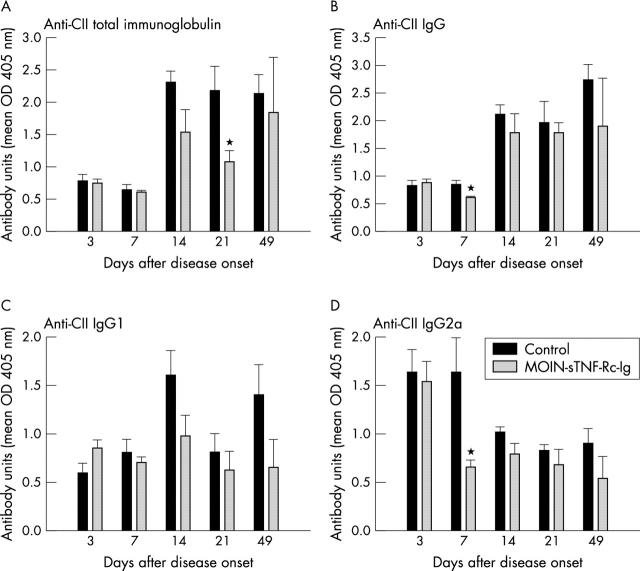
Figure 4.
Effect of periarticular delivery of MOIN-sTNF-Rc-Ig on the antibody profile to bovine CII. At arthritis onset mice received single periarticular injections of MOIN-sTNF-Rc-Ig or media into the affected arthritic paw. Serum samples were collected 3, 7, 14, 21, and 49 days after arthritis onset. Titres of antibodies to bovine CII were measured by ELISA. Each column represents the anti-bovine CII (A) immunoglobulin; (B) IgG; (C) IgG1; and (D) IgG2a levels in the treated or control sera at these times. Values are means (SEM) for the total number of mice in each group for each time. n=5 for both the media and MOIN-sTNF-Rc-Ig treated mice. *p<0.05.
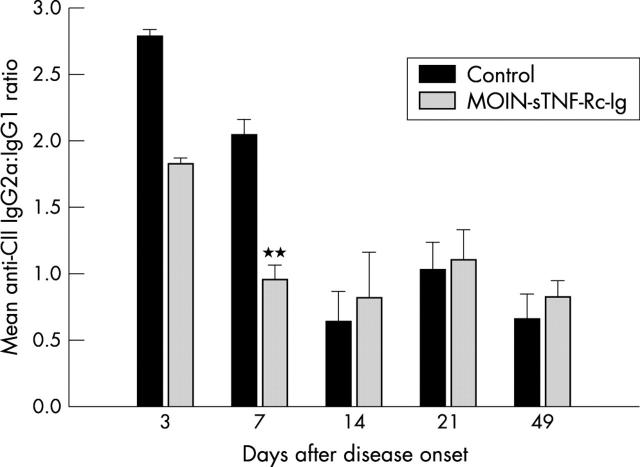
Figure 5.
Effect of a single periarticular delivery of MOIN-sTNF-Rc-Ig on the antibovine CII IgG2a:IgG1 ratio. Titres of anti-bovine CII IgG1 and IgG2a were measured by ELISA. The anti-CII IgG2a:IgG1 ratio 3, 7, 14, 21, and 49 days after disease onset is represented. Each column represents the mean ratios (SEM) for the total number of mice in each group for each time. n = 5 for both the media and MOIN-sTNF-Rc-Ig treated mice. **p<0.005.
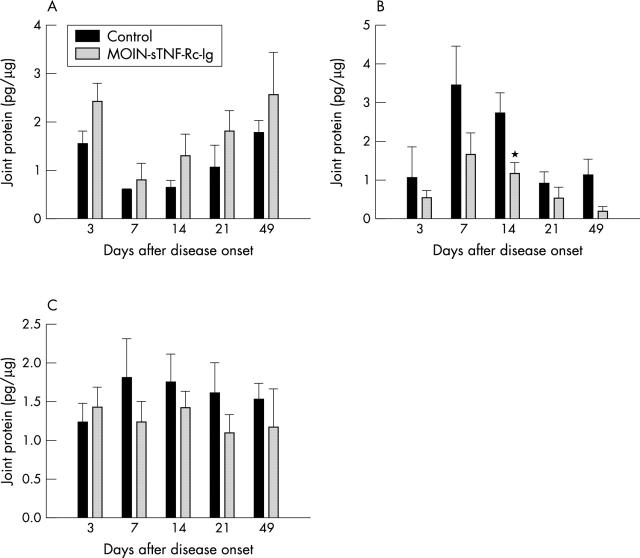
Figure 6.
Effect of a single periarticular delivery of MOIN-sTNF-Rc-Ig on the local TNFα levels. Local TNFα levels present in tissue lysates obtained from (A) injected (B) ipsilateral paws of control and MOIN-sTNF-Rc-Ig treated mice, harvested at 3, 7, 14, 21, and 49 days after disease onset were measured by ELISA. Contralateral joints showed results very similar to the ipsilateral joints. The cumulative levels of local TNFα expression in the injected, ipsilateral, and contralateral joints of the treated and control animals are represented in (C). Each column represents the mean (SEM) TNFα levels for the total number of mice in each group for each time. n = 5 for both the media and MOIN-sTNF-Rc-Ig treated mice. *p<0.05.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Brown C. B., Kaushansky K., Firestein G. S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [PubMed] [Google Scholar]
- Apparailly F., Verwaerde C., Jacquet C., Auriault C., Sany J., Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998 Jun 1;160(11):5213–5220. [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Wei B. Y., Hillman K., Luthra H. S., David C. S. Immunosuppression of collagen-induced arthritis in mice with an anti-IL-2 receptor antibody. J Immunol. 1988 Aug 15;141(4):1150–1154. [PubMed] [Google Scholar]
- Bendele A. M., Chlipala E. S., Scherrer J., Frazier J., Sennello G., Rich W. J., Edwards C. K., 3rd Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000 Dec;43(12):2648–2659. doi: 10.1002/1529-0131(200012)43:12<2648::AID-ANR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bendele A. M., McComb J., Gould T., Frazier J., Chlipala E. S., Seely J., Kieft G., Wolf J., Edwards C. K., 3rd Combination benefit of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) and dexamethasone or indomethacin in adjuvant arthritic rats. Inflamm Res. 1999 Aug;48(8):453–460. doi: 10.1007/s000110050486. [DOI] [PubMed] [Google Scholar]
- Bendele A. M., McComb J., Gould T., Frazier J., Chlipala E., Seely J., Kieft G., Edwards C. K., 3rd Effects of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) alone and in combination with methotrexate in adjuvant arthritic rats. Clin Exp Rheumatol. 1999 Sep-Oct;17(5):553–560. [PubMed] [Google Scholar]
- Boissier M. C., Chiocchia G., Bessis N., Hajnal J., Garotta G., Nicoletti F., Fournier C. Biphasic effect of interferon-gamma in murine collagen-induced arthritis. Eur J Immunol. 1995 May;25(5):1184–1190. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- Brand D. D., Marion T. N., Myers L. K., Rosloniec E. F., Watson W. C., Stuart J. M., Kang A. H. Autoantibodies to murine type II collagen in collagen-induced arthritis: a comparison of susceptible and nonsusceptible strains. J Immunol. 1996 Dec 1;157(11):5178–5184. [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Butler D. M., Maini R. N., Feldmann M., Brennan F. M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995 Jul-Dec;6(4):225–230. [PubMed] [Google Scholar]
- Chabaud M., Page G., Miossec P. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol. 2001 Nov 15;167(10):6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- Charles P., Elliott M. J., Davis D., Potter A., Kalden J. R., Antoni C., Breedveld F. C., Smolen J. S., Eberl G., deWoody K. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999 Aug 1;163(3):1521–1528. [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Londei M. Induction of Th2 cytokines and control of collagen-induced arthritis by nondepleting anti-CD4 Abs. J Immunol. 1996 Sep 15;157(6):2685–2689. [PubMed] [Google Scholar]
- Evans C., Robbins P. D. Prospects for treating arthritis by gene therapy. J Rheumatol. 1994 May;21(5):779–782. [PubMed] [Google Scholar]
- Gabay C., Marinova-Mutafchieva L., Williams R. O., Gigley J. P., Butler D. M., Feldmann M., Arend W. P. Increased production of intracellular interleukin-1 receptor antagonist type I in the synovium of mice with collagen-induced arthritis: a possible role in the resolution of arthritis. Arthritis Rheum. 2001 Feb;44(2):451–462. doi: 10.1002/1529-0131(200102)44:2<451::AID-ANR64>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gay S., Gay R. E., Koopman W. J. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis. 1993 Mar;52 (Suppl 1):S39–S47. doi: 10.1136/ard.52.suppl_1.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghivizzani S. C., Lechman E. R., Kang R., Tio C., Kolls J., Evans C. H., Robbins P. D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C., Brennan F. M., Chantry D., Turner M., Maini R. N., Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur J Immunol. 1991 Oct;21(10):2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Horsfall A. C., Butler D. M., Marinova L., Warden P. J., Williams R. O., Maini R. N., Feldmann M. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997 Dec 1;159(11):5687–5696. [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., Saxne T., van De Loo F. A., Heinegard D., van Den Berg W. B. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999 Nov 1;163(9):5049–5055. [PubMed] [Google Scholar]
- Kim S. H., Yu S. S., Park J. S., Robbins P. D., An C. S., Kim S. Construction of retroviral vectors with improved safety, gene expression, and versatility. J Virol. 1998 Feb;72(2):994–1004. doi: 10.1128/jvi.72.2.994-1004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mageed R. A., Adams G., Woodrow D., Podhajcer O. L., Chernajovsky Y. Prevention of collagen-induced arthritis by gene delivery of soluble p75 tumour necrosis factor receptor. Gene Ther. 1998 Dec;5(12):1584–1592. doi: 10.1038/sj.gt.3300785. [DOI] [PubMed] [Google Scholar]
- Mauri C., Williams R. O., Walmsley M., Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996 Jul;26(7):1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- Mittereder N., March K. L., Trapnell B. C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996 Nov;70(11):7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland L. W., Pratt P. W., Bucy R. P., Jackson B. S., Feldman J. W., Koopman W. J. Treatment of refractory rheumatoid arthritis with a chimeric anti-CD4 monoclonal antibody. Long-term followup of CD4+ T cell counts. Arthritis Rheum. 1994 Jun;37(6):834–838. doi: 10.1002/art.1780370610. [DOI] [PubMed] [Google Scholar]
- Myers L. K., Rosloniec E. F., Cremer M. A., Kang A. H. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61(19):1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U., Roberts C. R., Franklin B. N., Gay R. E., Robbins P. D., Evans C. H., Gay S. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J Immunol. 1997 Apr 1;158(7):3492–3498. [PubMed] [Google Scholar]
- Peppel K., Crawford D., Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991 Dec 1;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke K., Carlson D. G., Gitter B. D., Butler L. D. Role of interleukin 1 and interleukin 2 in rat and mouse arthritis models. J Immunol. 1986 Jun 1;136(11):4085–4091. [PubMed] [Google Scholar]
- Quattrocchi E., Walmsley M., Browne K., Williams R. O., Marinova-Mutafchieva L., Buurman W., Butler D. M., Feldmann M. Paradoxical effects of adenovirus-mediated blockade of TNF activity in murine collagen-induced arthritis. J Immunol. 1999 Jul 15;163(2):1000–1009. [PubMed] [Google Scholar]
- Schulze-Koops H., Davis L. S., Haverty T. P., Wacholtz M. C., Lipsky P. E. Reduction of Th1 cell activity in the peripheral circulation of patients with rheumatoid arthritis after treatment with a non-depleting humanized monoclonal antibody to CD4. J Rheumatol. 1998 Nov;25(11):2065–2076. [PubMed] [Google Scholar]
- Sewell K. L., Trentham D. E. Pathogenesis of rheumatoid arthritis. Lancet. 1993 Jan 30;341(8840):283–286. doi: 10.1016/0140-6736(93)92627-6. [DOI] [PubMed] [Google Scholar]
- Skapenko A., Wendler J., Lipsky P. E., Kalden J. R., Schulze-Koops H. Altered memory T cell differentiation in patients with early rheumatoid arthritis. J Immunol. 1999 Jul 1;163(1):491–499. [PubMed] [Google Scholar]
- Smith T. A., Mehaffey M. G., Kayda D. B., Saunders J. M., Yei S., Trapnell B. C., McClelland A., Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993 Dec;5(4):397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Tomoda K., Yoo T. J., Townes A. S., Kang A. H. Serum transfer of collagen-induced arthritis. II. Identification and localization of autoantibody to type II collagen in donor and recipient rats. Arthritis Rheum. 1983 Oct;26(10):1237–1244. doi: 10.1002/art.1780261011. [DOI] [PubMed] [Google Scholar]
- Takagi N., Mihara M., Moriya Y., Nishimoto N., Yoshizaki K., Kishimoto T., Takeda Y., Ohsugi Y. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998 Dec;41(12):2117–2121. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Whalen J. D., Lechman E. L., Carlos C. A., Weiss K., Kovesdi I., Glorioso J. C., Robbins P. D., Evans C. H. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol. 1999 Mar 15;162(6):3625–3632. [PubMed] [Google Scholar]
- Wooley P. H., Chapedelaine J. M. Immunogenetics of collagen-induced arthritis. Crit Rev Immunol. 1987;8(1):1–22. [PubMed] [Google Scholar]
- Wooley P. H., Dutcher J., Widmer M. B., Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993 Dec 1;151(11):6602–6607. [PubMed] [Google Scholar]
- Wooley P. H., Whalen J. D. The influence of superoxide scavenging compound CTC 23 on type II collagen-induced arthritis in mice. Agents Actions. 1992 Mar;35(3-4):273–279. doi: 10.1007/BF01997511. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]