Abstract
Objective: To investigate the distribution of peripheral B cell subpopulations of children with active and inactive systemic lupus erythematosus (SLE) compared with healthy controls.
Methods: Peripheral B cell subpopulations of 11 children with SLE (6 with active and 5 with inactive disease) and 14 age matched normal healthy children were analysed. Active disease was diagnosed in children with a flare of SLE, who received treatment by IV cyclophosphamide or IV methylprednisolone pulse to control the disease. Additionally, the peripheral B cells of the children with SLE were compared with those of 13 consecutive patients with adult onset SLE.
Results: No major difference was found in the frequency and total number of CD27-/CD19+ naïve B cells and CD27+/CD19+ memory B cells between patients with active and inactive lupus and healthy controls, but there was a significant increase in CD27high expressing plasma blasts in patients with active SLE. These cells coexpress CD38+, HLA-DRdim, surface Iglow and lack the expression of CD20 but are clearly positive for intracellular Ig, indicative of early plasma cells. Most CD138+ cells coexpress CD27high/CD19+. The enhanced frequency of peripheral plasma blasts in children with active SLE is consistent with previous findings in adult patients with SLE, whereas a relative predominance of CD27+ memory B cells was only identified in the adult patients.
Conclusions: Profound abnormalities in the distribution of B cell compartments are more pronounced in older patients with SLE, but an enhanced frequency and cell number of peripheral plasma blasts is characteristic of both diseases during active stages. Thus detection of CD27high plasma blasts significantly correlates with active lupus in both children and adults.
Full Text
The Full Text of this article is available as a PDF (483.6 KB).
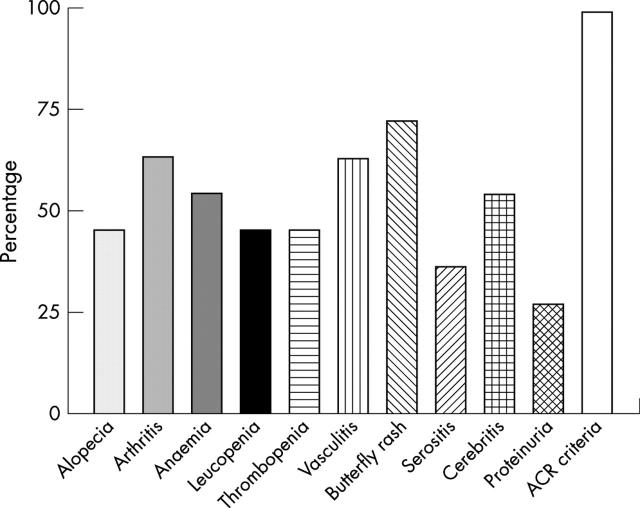
Figure 1.
Frequency of clinical manifestations of the children with SLE analysed in the current study. All analysed children showed at least four different clinical manifestations classifying them according to the 1982 revised ACR criteria.
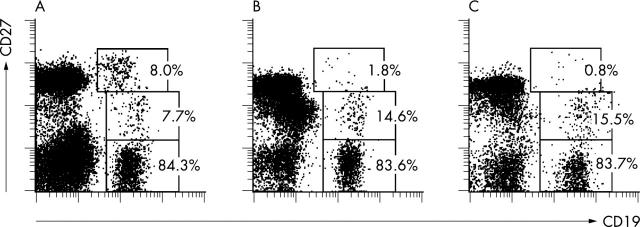
Figure 2.
Expression of CD27 on CD19+ peripheral B cells from children with SLE and from a normal healthy donor. Viable PBMC were gated for analysis according to light scatter and exclusion of propidium iodide. Staining with CD19 bio/SA-PE versus CD27 Cy5 is shown for (A) a child with active SLE (donor No 6); (B) a child with inactive SLE (donor No 3); and (C) a healthy child recruited outside the hospital (donor No 12). Gates for the statistical evaluation of the frequency of CD27-, CD27+, and CD27high among CD19+ peripheral B cells are indicated. The respective frequencies are indicated.
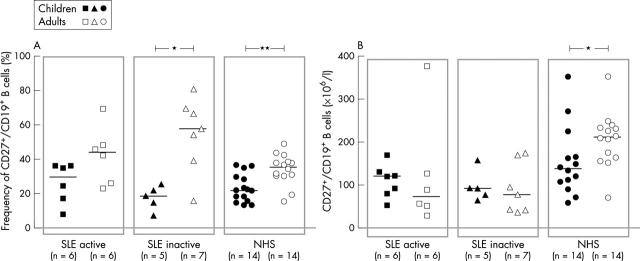
Figure 7.
Comparison of the (A) frequency and (B) total numbers of CD27+ peripheral memory B cells in children and adults from a previous study of patients with active and inactive SLE as well as in their respective healthy controls. The frequencies were determined by cytometric analysis as shown in fig 2. The median values are indicated. *p<0.05; **p<0.01.
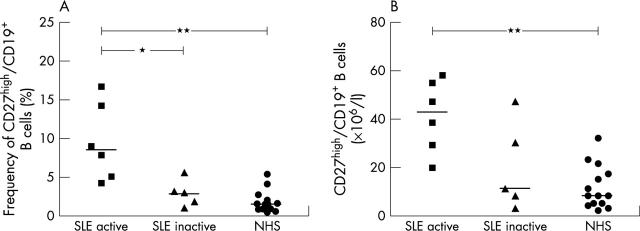
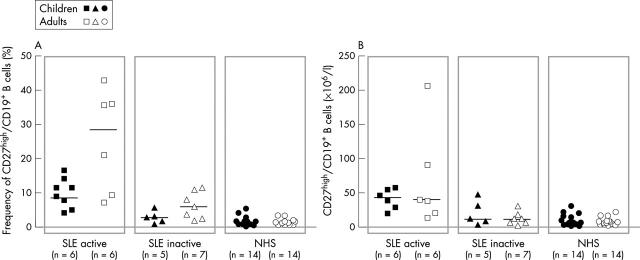
Figure 3.
Frequency (A) and total number (B) of CD27high peripheral plasma cells in patients with active and inactive SLE and in a control group of healthy subjects. The frequencies were determined by cytometric analysis as shown in fig 2. The median value is indicated. *p<0.05; **p<0.01.
Figure 4.
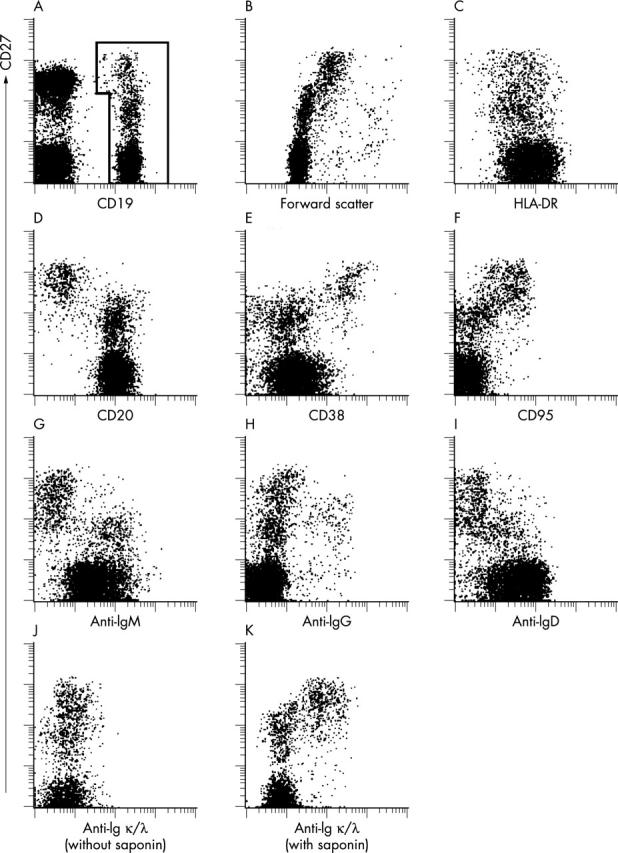
Cytometric characterisation of peripheral CD19+ B cells in a child with SLE (donor No 2). (A) Viable peripheral mononuclear cells of a patient with SLE, gated according to scatter and propidium iodide exclusion, were tested for CD19 and CD27 expression. CD19+ B cells were gated for further analysis as indicated. (B) Staining of CD27 versus forward light scatter to analyse differences in cell size. (C-I) CD19+ B cells, as gated in (A), were counterstained for HLA-DR, CD20, CD38, CD95, IgM, IgG, or IgD, respectively. Formaldehyde fixed cells were gated according to scatter and CD19-PE staining and counterstained for CD27 and for Igκ and Igλ light chains, without (J) or with (K) permeabilisation of the cell membrane by the use of saponin.
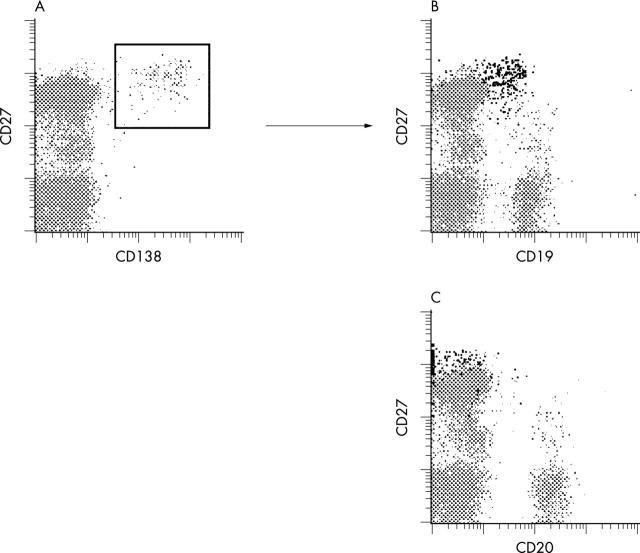
Figure 5.
Cytometric characterisation of peripheral CD138+ cells in a child with a lupus flare (donor No 6). (A) Viable peripheral mononuclear cells of a patient with a lupus flare were gated according to scatter and propidium iodide exclusion and stained for CD138-PE, CD27-Cy5, and either CD19-FITC or CD20-FITC, respectively. Coexpression of CD27 and CD19 (B) or CD20 (C), respectively, on CD138+ cells, as gated in (A), is shown in a two colour flow cytometric analysis. CD138+ coexpressing cells in (B) and (C) are depicted in black.
Figure 8.
Comparison of the (A) frequency and (B) total numbers of CD27high peripheral plasma cells in children and adults from a previous study of patients with active and inactive SLE as well as in their respective healthy controls. The frequencies were determined by cytometric analysis as shown in fig 2. The median values are indicated. No significant difference was found between the children and adults with SLE.
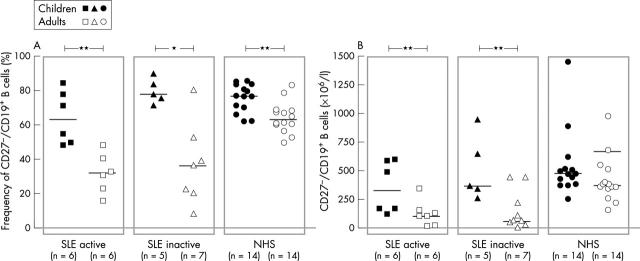
Figure 6.
Comparison of the (A) frequency and (B) total numbers of CD27- peripheral naïve B cells in children and adults from a previous study of patients with active and inactive SLE as well as their respective healthy controls. The frequencies were determined by cytometric analysis as shown in fig 2. The median values are indicated. *p<0.05; **p<0.01.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agematsu K., Nagumo H., Oguchi Y., Nakazawa T., Fukushima K., Yasui K., Ito S., Kobata T., Morimoto C., Komiyama A. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 1998 Jan 1;91(1):173–180. [PubMed] [Google Scholar]
- Agematsu K., Nagumo H., Shinozaki K., Hokibara S., Yasui K., Terada K., Kawamura N., Toba T., Nonoyama S., Ochs H. D. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998 Aug 15;102(4):853–860. doi: 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agematsu K., Nagumo H., Yang F. C., Nakazawa T., Fukushima K., Ito S., Sugita K., Mori T., Kobata T., Morimoto C. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol. 1997 Aug;27(8):2073–2079. doi: 10.1002/eji.1830270835. [DOI] [PubMed] [Google Scholar]
- Arce E., Jackson D. G., Gill M. A., Bennett L. B., Banchereau J., Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001 Aug 15;167(4):2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- Bourne T., Zukowska-Cooper M., Salaman M. R., Seifert M. H., Isenberg D. A. Spontaneous immunoglobulin-producing capacity of cultures from lupus patients and normal donors following depletion of cells expressing CD19 or CD38. Clin Exp Immunol. 1998 Mar;111(3):611–616. doi: 10.1046/j.1365-2249.1998.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O. T., Madaio M. P., Shlomchik M. J. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999 Jun;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg W. W., Finkelman F. D., Lipsky P. E. Circulating and pokeweed mitogen-induced immunoglobulin-secreting cells in systemic lupus erythematosus. Clin Exp Immunol. 1979 Jan;35(1):76–88. [PMC free article] [PubMed] [Google Scholar]
- Harley J. B., Moser K. L., Gaffney P. M., Behrens T. W. The genetics of human systemic lupus erythematosus. Curr Opin Immunol. 1998 Dec;10(6):690–696. doi: 10.1016/s0952-7915(98)80090-3. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997 Feb 15;89(4):1288–1298. [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Klein U., Rajewsky K., Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998 Nov 2;188(9):1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Arpin C. Germinal center development. Immunol Rev. 1997 Apr;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Arpin C., de Bouteiller O., Guret C., Banchereau J., Martinez-Valdez H., Lebecque S. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Semin Immunol. 1996 Jun;8(3):169–177. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin Immunol. 1997 Aug;9(4):235–240. doi: 10.1006/smim.1997.0080. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Liu Y. J., Johnson G. D. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992 Apr;126:143–161. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud M. S., Fujii R., Ishikawa H., Kawano M. M. Enforced CD19 expression leads to growth inhibition and reduced tumorigenicity. Blood. 1999 Nov 15;94(10):3551–3558. [PubMed] [Google Scholar]
- Morton S. J., Powell R. J. Management of systemic lupus erythematosus (SLE). Clin Exp Allergy. 2001 May;31(5):686–693. doi: 10.1046/j.1365-2222.2001.01123.x. [DOI] [PubMed] [Google Scholar]
- Nagumo H., Agematsu K., Shinozaki K., Hokibara S., Ito S., Takamoto M., Nikaido T., Yasui K., Uehara Y., Yachie A. CD27/CD70 interaction augments IgE secretion by promoting the differentiation of memory B cells into plasma cells. J Immunol. 1998 Dec 15;161(12):6496–6502. [PubMed] [Google Scholar]
- Odendahl M., Jacobi A., Hansen A., Feist E., Hiepe F., Burmester G. R., Lipsky P. E., Radbruch A., Dörner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000 Nov 15;165(10):5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- Schwab J., Lukowsky A., Volk H. D., Peter H. H., Melchers I. Precursor frequencies for DNA-specific B lymphocytes in patients with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1994 Jun;96(3):450–457. doi: 10.1111/j.1365-2249.1994.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk P. E., Horst G., Van Der Gun B. T., Limburg P. C., Kallenberg C. G. Anti-dsDNA production coincides with concurrent B and T cell activation during development of active disease in systemic lupus erythematosus (SLE). Clin Exp Immunol. 1996 Jun;104(3):446–453. doi: 10.1046/j.1365-2249.1996.44754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk P. E., vd Gun B. T., Limburg P. C., Kallenberg C. G. B cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to levels of anti-dsDNA, nor to concurrent T cell activation. Clin Exp Immunol. 1993 Jul;93(1):39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann N Y Acad Sci. 1997 Apr 5;815:1–14. doi: 10.1111/j.1749-6632.1997.tb52040.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tangye S. G., Liu Y. J., Aversa G., Phillips J. H., de Vries J. E. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998 Nov 2;188(9):1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Han S., Takahashi Y., Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997 Dec;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]