Abstract
Objectives: To determine if treatment with licofelone, a combined 5-lipoxygenase and cyclo-oxygenase inhibitor, in vivo in experimental dog osteoarthritis can modify bone cell metabolism in long term in vitro subchondral osteoblast cell cultures (Ob).
Methods: Group 1 received sectioning of the anterior cruciate ligament (ACL) of the right knee with no active treatment (placebo group). Groups 2 and 3 received sectioning of the ACL of the right knee, and were given licofelone (2.5 or 5.0 mg/kg daily by mouth, respectively) for eight weeks beginning the day after surgery. Primary Ob were prepared from the subchondral bone plate. Levels of phenotypic markers (alkaline phosphatase activity, osteocalcin release), and urokinase plasminogen activator (uPA) and insulin-like growth factor-1 (IGF-I) levels, were evaluated in each group. Lastly, prostaglandin E2 (PGE2) and leucotriene B4 levels were evaluated.
Results: No significant differences in alkaline phosphatase activity or osteocalcin release from Ob between the three groups, under either basal or 1,25(OH)2D3 induction were seen. In contrast, treatment with licofelone reduced uPA and IGF-I levels in Ob. PGE2 levels, which were still raised in the placebo group, were decreased sharply by licofelone. A relationship was found between licofelone treatment and either the reduction in the size of lesions on tibial plateaus or the levels of uPA, IGF-I, or PGE2.
Conclusions: Licofelone treatment prevents and/or delays the abnormal metabolism of subchondral osteoblasts in this model. Licofelone reduced PGE2 levels after long term Ob, suggesting that the reduction in uPA and IGF-I levels is linked, at least in part, to this reduction.
Full Text
The Full Text of this article is available as a PDF (282.7 KB).
Figure 1 .
Evaluation of alkaline phosphatase activity of in vitro osteoblasts after in vivo treatment with placebo or with 2.5 mg/kg or 5.0 mg/kg licofelone daily for eight weeks of dogs with OA. Confluent primary osteoblasts were incubated for their last two days of culture with Ham's F12/DMEM containing 2% charcoal treated FBS in the presence or absence of 50 nM 1,25(OH)2D3. Alkaline phosphatase activity was determined by the hydrolysis of p-nitrophenyl phosphate into p-nitrophenol. Values are the mean (SEM) of seven cell cultures for each experimental group. No statistical differences were seen between the groups.
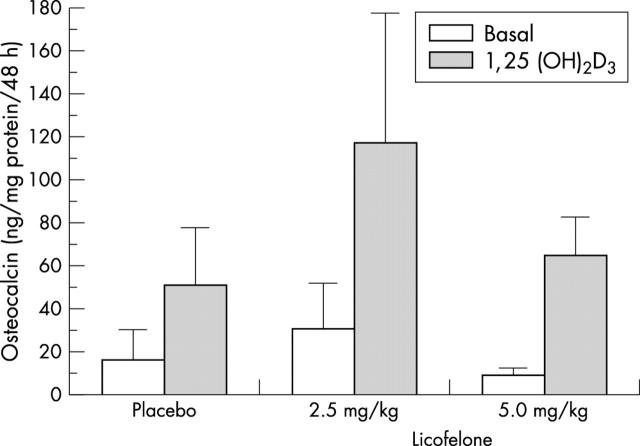
Figure 2 .
Evaluation of osteocalcin release of in vitro osteoblasts after in vivo treatment with placebo or with 2.5 mg/kg or 5.0 mg/kg licofelone daily for eight weeks of dogs with OA. Confluent primary osteoblasts were prepared with the same experimental protocol as in fig 1. Osteocalcin release was determined on aliquots of conditioned media by radioimmunoassay. Values are the mean (SEM) of seven cell cultures for each group. No statistical differences were seen between the groups.
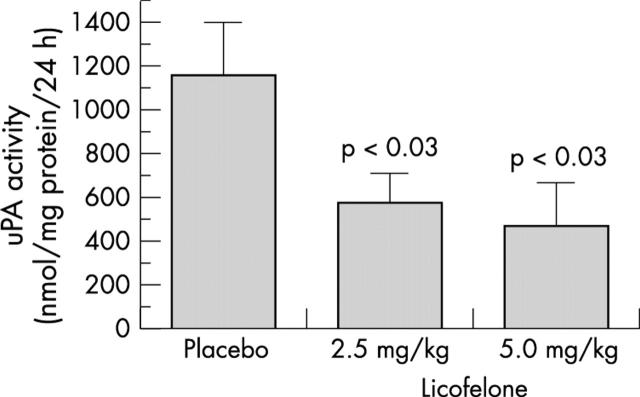
Figure 3 .
Evaluation of urokinase plasminogen activator (uPA) activity in dog osteoblasts. Dogs were treated with placebo or with 2.5 mg/kg or 5.0 mg/kg licofelone for eight weeks before the preparation of primary osteoblast cell cultures. Confluent primary dog osteoblasts were incubated for their last two days of culture in the presence of Ham's F12/DMEM without serum containing 1% ITS mix. uPA activity was determined on aliquots of conditioned media using a chromogenic substrate. Values are the mean (SEM) of seven cell cultures for each group.
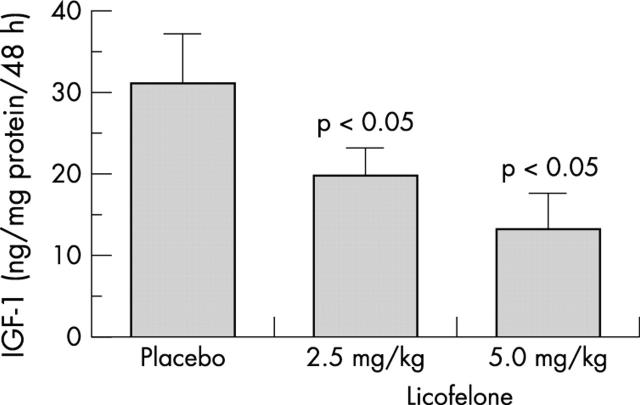
Figure 4 .
Evaluation of insulin-like growth factor-I (IGF-I) levels in dog osteoblasts. The same experimental protocol as in fig 3 was performed. IGF-I levels were determined by a selective ELISA. Values are the mean (SEM) of seven cell cultures for each group.
Figure 5 .
Evaluation of prostaglandin E2 (PGE2) levels in dog osteoblasts. The same experimental protocol as in fig 3 was performed. PGE2 was determined on aliquots by a selective ELISA. Values are the mean (SEM) of seven cell cultures for each group.
Figure 6 .
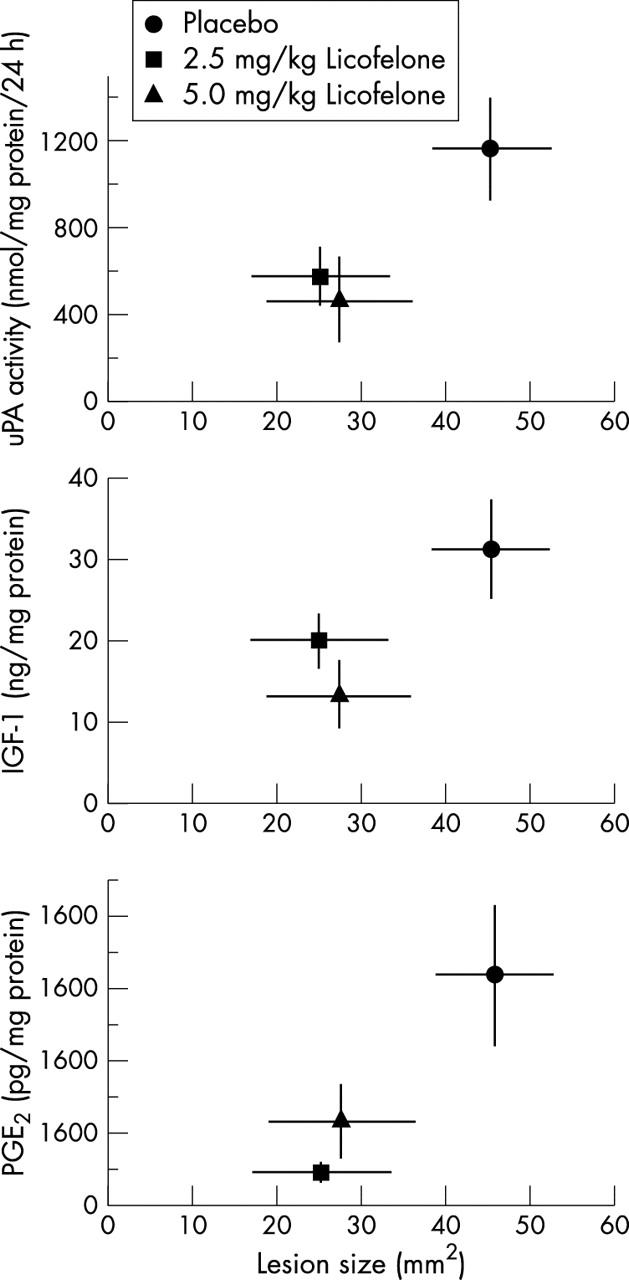
Relationship between the size (surface, mm2) of macroscopic lesions on the tibial plateau and uPA activity, IGF-I or PGE2 levels in dog osteoblasts from dogs treated with placebo or with 2.5 mg/kg or 5.0 mg/kg licofelone daily for eight weeks before the preparation of primary osteoblasts. Individual values for uPA activity are derived from fig 3, IGF-I from fig 4, and PGE2 from fig 5. Values are the mean (SEM) of seven cell cultures for each group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey Allen J., Sims Trevor J., Knott Lynda. Phenotypic expression of osteoblast collagen in osteoarthritic bone: production of type I homotrimer. Int J Biochem Cell Biol. 2002 Feb;34(2):176–182. doi: 10.1016/s1357-2725(01)00107-8. [DOI] [PubMed] [Google Scholar]
- Brandt K. D. Insights into the natural history of osteoarthritis provided by the cruciate-deficient dog. An animal model of osteoarthritis. Ann N Y Acad Sci. 1994 Sep 6;732:199–205. doi: 10.1111/j.1749-6632.1994.tb24735.x. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Myers S. L., Burr D., Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991 Dec;34(12):1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- Carlson C. S., Loeser R. F., Purser C. B., Gardin J. F., Jerome C. P. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996 Sep;11(9):1209–1217. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- Dedrick D. K., Goldstein S. A., Brandt K. D., O'Connor B. L., Goulet R. W., Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993 Oct;36(10):1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- Dedrick D. K., Goulet R. W., O'Connor B. L., Brandt K. D. Preliminary report: increased porosity of the subchondral plate in an accelerated canine model of osteoarthritis. Osteoarthritis Cartilage. 1997 Jan;5(1):71–74. doi: 10.1016/s1063-4584(97)80033-7. [DOI] [PubMed] [Google Scholar]
- Dequeker J., Mohan S., Finkelman R. D., Aerssens J., Baylink D. J. Generalized osteoarthritis associated with increased insulin-like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis Rheum. 1993 Dec;36(12):1702–1708. doi: 10.1002/art.1780361209. [DOI] [PubMed] [Google Scholar]
- Fernandes J. C., Martel-Pelletier J., Otterness I. G., Lopez-Anaya A., Mineau F., Tardif G., Pelletier J. P. Effects of tenidap on canine experimental osteoarthritis. I. Morphologic and metalloprotease analysis. Arthritis Rheum. 1995 Sep;38(9):1290–1303. doi: 10.1002/art.1780380918. [DOI] [PubMed] [Google Scholar]
- Gevers G., Dequeker J. Collagen and non-collagenous protein content (osteocalcin, sialoprotein, proteoglycan) in the iliac crest bone and serum osteocalcin in women with and without hand osteoarthritis. Coll Relat Res. 1987 Dec;7(6):435–442. doi: 10.1016/s0174-173x(87)80041-9. [DOI] [PubMed] [Google Scholar]
- Grynpas M. D., Alpert B., Katz I., Lieberman I., Pritzker K. P. Subchondral bone in osteoarthritis. Calcif Tissue Int. 1991 Jul;49(1):20–26. doi: 10.1007/BF02555898. [DOI] [PubMed] [Google Scholar]
- Gundberg C. M., Hauschka P. V., Lian J. B., Gallop P. M. Osteocalcin: isolation, characterization, and detection. Methods Enzymol. 1984;107:516–544. doi: 10.1016/0076-6879(84)07036-1. [DOI] [PubMed] [Google Scholar]
- Hilal G., Martel-Pelletier J., Pelletier J. P., Duval N., Lajeunesse D. Abnormal regulation of urokinase plasminogen activator by insulin-like growth factor 1 in human osteoarthritic subchondral osteoblasts. Arthritis Rheum. 1999 Oct;42(10):2112–2122. doi: 10.1002/1529-0131(199910)42:10<2112::AID-ANR11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hilal G., Martel-Pelletier J., Pelletier J. P., Ranger P., Lajeunesse D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 1998 May;41(5):891–899. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hulth A. Does osteoarthrosis depend on growth of the mineralized layer of cartilage? Clin Orthop Relat Res. 1993 Feb;(287):19–24. [PubMed] [Google Scholar]
- Imhof H., Breitenseher M., Kainberger F., Rand T., Trattnig S. Importance of subchondral bone to articular cartilage in health and disease. Top Magn Reson Imaging. 1999 Jun;10(3):180–192. doi: 10.1097/00002142-199906000-00002. [DOI] [PubMed] [Google Scholar]
- Jovanovic D. V., Fernandes J. C., Martel-Pelletier J., Jolicoeur F. C., Reboul P., Laufer S., Tries S., Pelletier J. P. In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1beta synthesis. Arthritis Rheum. 2001 Oct;44(10):2320–2330. doi: 10.1002/1529-0131(200110)44:10<2320::aid-art394>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lajeunesse D., Busque L., Ménard P., Brunette M. G., Bonny Y. Demonstration of an osteoblast defect in two cases of human malignant osteopetrosis. Correction of the phenotype after bone marrow transplant. J Clin Invest. 1996 Oct 15;98(8):1835–1842. doi: 10.1172/JCI118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse D., Kiebzak G. M., Frondoza C., Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner. 1991 Sep;14(3):237–250. doi: 10.1016/0169-6009(91)90025-u. [DOI] [PubMed] [Google Scholar]
- Leprince P., Rogister B., Moonen G. A colorimetric assay for the simultaneous measurement of plasminogen activators and plasminogen activator inhibitors in serum-free conditioned media from cultured cells. Anal Biochem. 1989 Mar;177(2):341–346. doi: 10.1016/0003-2697(89)90063-8. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R. M. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J Bone Miner Res. 1997 Apr;12(4):641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- Li B., Aspden R. M. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997 Apr;56(4):247–254. doi: 10.1136/ard.56.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell J. P., Bailey A. J. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998 Apr 15;101(8):1596–1603. doi: 10.1172/JCI867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell J. P., Tarlton J. F., Bailey A. J. Biochemical evidence for altered subchondral bone collagen metabolism in osteoarthritis of the hip. Br J Rheumatol. 1997 Jan;36(1):16–19. doi: 10.1093/rheumatology/36.1.16. [DOI] [PubMed] [Google Scholar]
- Massicotte F., Lajeunesse D., Benderdour M., Pelletier J-P, Hilal G., Duval N., Martel-Pelletier J. Can altered production of interleukin-1beta, interleukin-6, transforming growth factor-beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage. 2002 Jun;10(6):491–500. doi: 10.1053/joca.2002.0528. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Raisz L. G., Canalis E. Prostaglandin E2 stimulates insulin-like growth factor I synthesis in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1991 Jun;128(6):2895–2900. doi: 10.1210/endo-128-6-2895. [DOI] [PubMed] [Google Scholar]
- Mital M. A., Millington P. F. Osseous pathway of nutrition to articular cartilage of the human femoral head. Lancet. 1970 Apr 18;1(7651):842–842. doi: 10.1016/s0140-6736(70)92443-8. [DOI] [PubMed] [Google Scholar]
- Mohan S., Bautista C. M., Herring S. J., Linkhart T. A., Baylink D. J. Development of valid methods to measure insulin-like growth factors-I and -II in bone cell-conditioned medium. Endocrinology. 1990 May;126(5):2534–2542. doi: 10.1210/endo-126-5-2534. [DOI] [PubMed] [Google Scholar]
- Paredes Yosabeth, Massicotte Frédéric, Pelletier Jean-Pierre, Martel-Pelletier Johanne, Laufer Stefan, Lajeunesse Daniel. Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum. 2002 Jul;46(7):1804–1812. doi: 10.1002/art.10357. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., DiBattista J. A., Raynauld J. P., Wilhelm S., Martel-Pelletier J. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Invest. 1995 May;72(5):578–586. [PubMed] [Google Scholar]
- Pelletier J. P., Jovanovic D., Fernandes J. C., Manning P., Connor J. R., Currie M. G., Di Battista J. A., Martel-Pelletier J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998 Jul;41(7):1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Lajeunesse D., Jovanovic D. V., Lascau-Coman V., Jolicoeur F. C., Hilal G., Fernandes J. C., Martel-Pelletier J. Carprofen simultaneously reduces progression of morphological changes in cartilage and subchondral bone in experimental dog osteoarthritis. J Rheumatol. 2000 Dec;27(12):2893–2902. [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Abramson S. B. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001 Jun;44(6):1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Mineau F., Raynauld J. P., Woessner J. F., Jr, Gunja-Smith Z., Martel-Pelletier J. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum. 1994 Mar;37(3):414–423. doi: 10.1002/art.1780370316. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Rose R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986 Dec;(213):34–40. [PubMed] [Google Scholar]
- Schouten J. S., Van den Ouweland F. A., Valkenburg H. A., Lamberts S. W. Insulin-like growth factor-1: a prognostic factor of knee osteoarthritis. Br J Rheumatol. 1993 Apr;32(4):274–280. doi: 10.1093/rheumatology/32.4.274. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Microcracks in the calcified layer of articular cartilage. Arch Pathol Lab Med. 1993 Feb;117(2):191–195. [PubMed] [Google Scholar]
- Westacott C. I., Webb G. R., Warnock M. G., Sims J. V., Elson C. J. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1997 Jul;40(7):1282–1291. doi: 10.1002/1529-0131(199707)40:7<1282::AID-ART13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., Glansbeek H. L., van der Kraan P. M., van den Berg W. B. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998 Sep;6(5):306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- van Beuningen H. M., van der Kraan P. M., Arntz O. J., van den Berg W. B. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994 Aug;71(2):279–290. [PubMed] [Google Scholar]
- van den Berg W. B. Growth factors in experimental osteoarthritis: transforming growth factor beta pathogenic? J Rheumatol Suppl. 1995 Feb;43:143–145. [PubMed] [Google Scholar]