Abstract
Objective: To investigate the indirect effects of anti-CD4 treatment on the functions of macrophages (CD4– in mice) in the acute and early chronic phase of mouse antigen induced arthritis (AIA).
Methods: C57BL/6 mice with AIA were treated intraperitoneally with the anti-CD4 mAb GK1.5 or control rat IgG on days –1, 0, 1, 3, 5, and 7. Proinflammatory cytokines (IL1ß, IL6, and TNFα) were quantified by sandwich ELISA in joint extracts, serum, and supernatants of ex vivo stimulated spleen/lymph node cells or peritoneal macrophages (+LPS/IFNγ). Nitric oxide (NO) levels in supernatants of ex vivo stimulated peritoneal macrophages were measured by the Griess reaction. Proteolytic activity in joint homogenates was analysed by gelatin, casein, and elastin zymography, and substrate assays.
Results: Anti-CD4 treatment significantly reduced joint swelling in acute (days 3, 5) and early chronic AIA (day 7) and diminished inflammation and destruction scores in late chronic AIA (day 21). On day 3, anti-CD4 treatment significantly reduced IL6 levels in all compartments. IL1ß was reduced in joint extracts, unaffected in serum or cells from lymphoid organs, and increased in stimulated peritoneal macrophages. TNFα was significantly increased in the joints, decreased in serum, and otherwise unchanged. NO production by stimulated peritoneal macrophages was significantly reduced by anti-CD4 treatment. Lower activity of matrix metalloproteinases and neutrophil elastase was seen in joint extracts of anti-CD4 treated animals than in IgG treated AIA controls.
Conclusion: CD4+ T cell directed treatment had strong local and systemic effects on macrophages. These indirect effects may contribute to the reduction of destructive mediators/joint destruction in AIA.
Full Text
The Full Text of this article is available as a PDF (214.9 KB).
Figure 1.
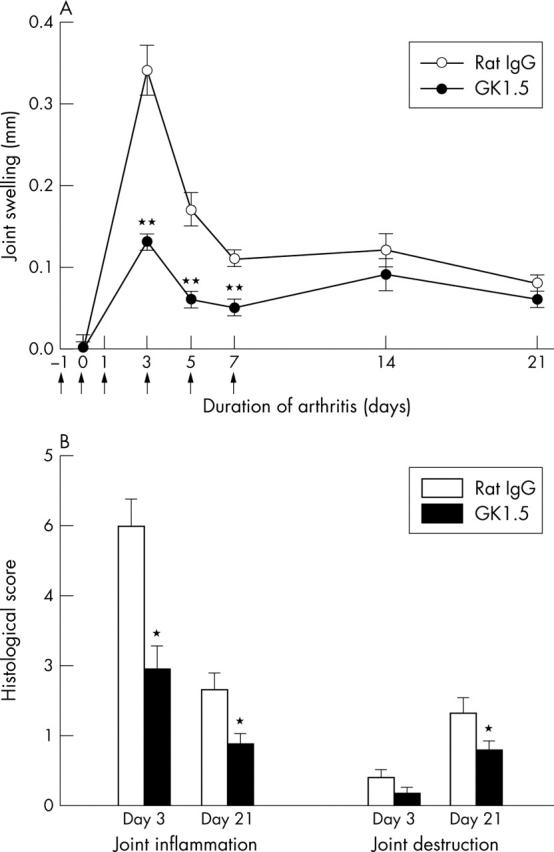
Joint swelling and histology of AIA. (A) Time course of joint swelling in AIA after treatment with the anti-CD4 mAb GK1.5 or control rat IgG. Results are expressed as means (SEM) of 10 individual animals in each group. Arrows indicate the days of treatment (days –1, 0, 1, 3, 5, and 7). (B) Histological score of joint inflammation and joint destruction in the acute phase (day 3) and the chronic phase (day 21) of AIA. Results are expressed as means (SEM) of 10 individual animals in each group. *p⩽0.05, **p⩽0.01 in comparison with IgG treated controls.
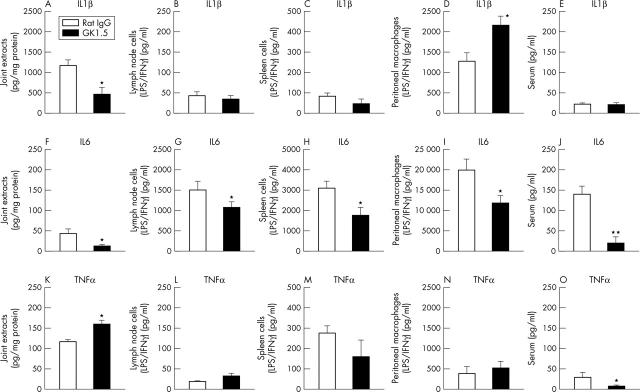
Figure 2.
Cytokine levels on day 3 of AIA. Concentrations of IL1ß (A, B, C, D, E), IL6 (F, G, H, I, J), and TNFα (K, L, M, N, O) in joint extracts, supernatants of stimulated lymph node cells, spleen cells, or peritoneal macrophages, as well as serum of GK1.5 treated or rat IgG treated mice, as determined by sandwich ELISA. Results are expressed as means (SEM) (n = 6 for each group). *p⩽0.05, **p⩽0.01 in comparison with IgG treated controls.
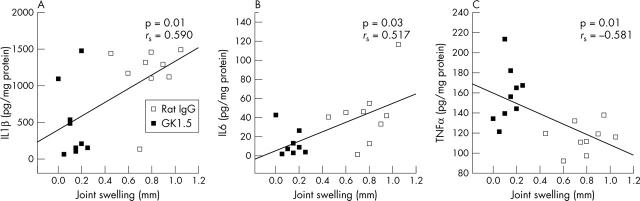
Figure 3.
Correlations between cytokine levels in joint extracts and joint swelling on day 3 of AIA. Correlations (Spearman rank test) between joint IL1ß, IL6, and TNFα levels and joint swelling of individual mice treated with GK1.5 or rat IgG (total of n = 18 for each cytokine).
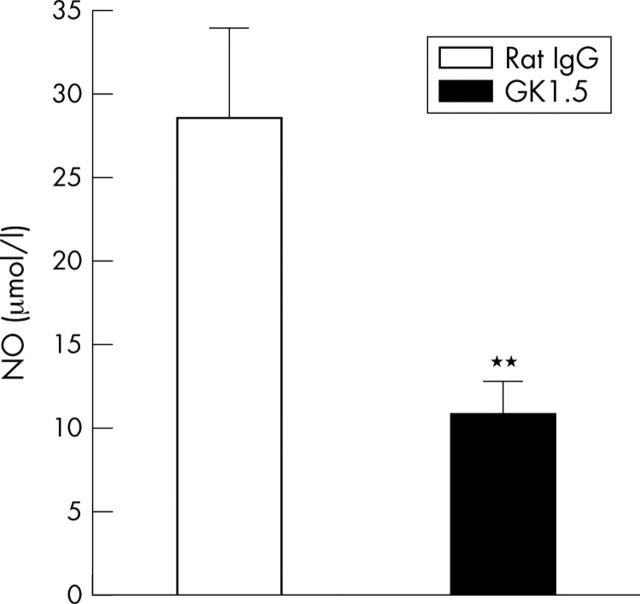
Figure 4.
NO levels on day 3 of AIA. Levels of NO in culture supernatants of LPS/IFNγ stimulated peritoneal macrophages from GK1.5 treated or rat IgG treated mice. Results are expressed as means (SEM) (n = 6 for each group). **p⩽0.01 in comparison with IgG treated controls.
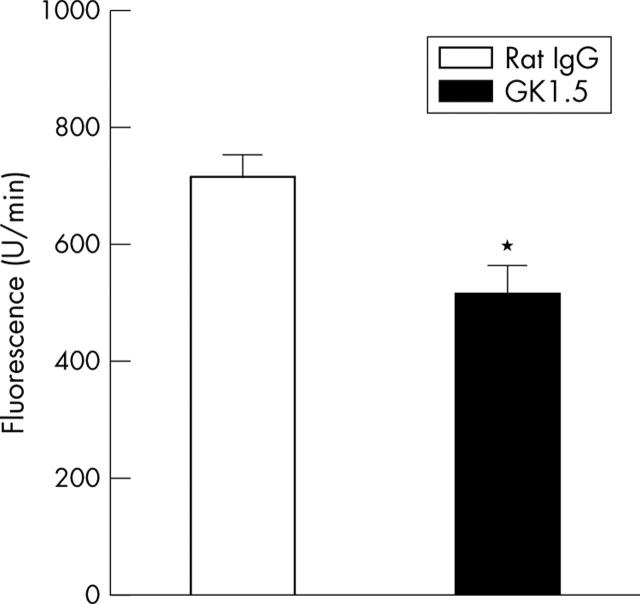
Figure 5.
"Total" MMP activity in joint extracts on day 3 of AIA. Total activity of MMP was determined by cleavage of the peptide MCA-Pro-Leu-Gly-Leu-DAP (DNP-Ala-Arg-NH2), resulting in an increase of fluorescence. Results are expressed as means (SEM) (n = 8 for each group). *p⩽0.05 in comparison with IgG treated controls.
Figure 6.
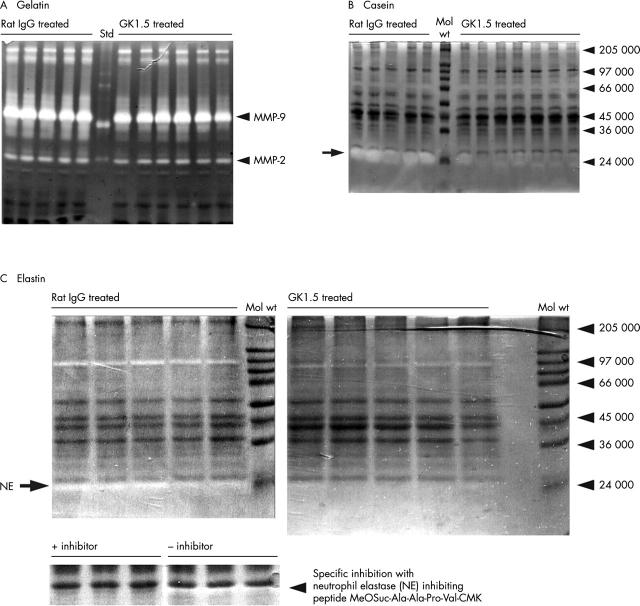
Zymography of joint extracts on day 3 of AIA. Zymography of equal amounts (protein content) of joint extracts from GK1.5 treated or rat IgG treated mice using (A) gelatin, (B) casein, or (C) elastin as a substrate. Markers of molecular weight (Mol wt) or the positions of recombinant human MMP standards (Std) are indicated on the right. Elastase activity in (C) was blocked by the specific inhibitor MeOSuc-Ala-Ala-Pro-Val-CMK.
Figure 7.
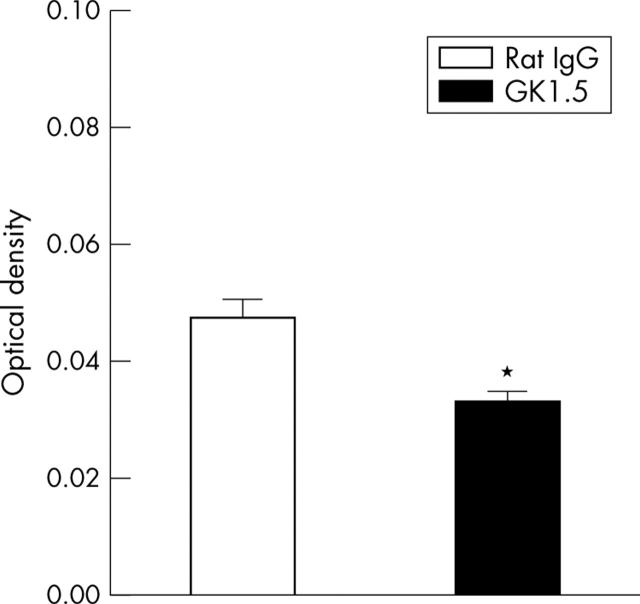
Elastase activity in joint extracts on day 3 of AIA. Activity of neutrophil elastase was determined by cleavage of the elastase-specific, synthetic substrate MeOSuc-Ala-Ala-Pro-Val-pNA, resulting in an increase of absorbance. Results are expressed as means (SEM) (n = 8 for each group). *p⩽0.05 in comparison with IgG treated controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsalameh S., Winter K., Al-Ward R., Wendler J., Kalden J. R., Kinne R. W. Distribution of TNF-alpha, TNF-R55 and TNF-R75 in the rheumatoid synovial membrane: TNF receptors are localized preferentially in the lining layer; TNF-alpha is distributed mainly in the vicinity of TNF receptors in the deeper layers. Scand J Immunol. 1999 Mar;49(3):278–285. doi: 10.1046/j.1365-3083.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Alsalameh Saifeddin, Amin Rayya J., Kunisch Elke, Jasin Hugo E., Kinne Raimund W. Preferential induction of prodestructive matrix metalloproteinase-1 and proinflammatory interleukin 6 and prostaglandin E2 in rheumatoid arthritis synovial fibroblasts via tumor necrosis factor receptor-55. J Rheumatol. 2003 Aug;30(8):1680–1690. [PubMed] [Google Scholar]
- Borghaei R. C., Rawlings P. L., Jr, Mochan E. Interleukin-4 suppression of interleukin-1-induced transcription of collagenase (MMP-1) and stromelysin 1 (MMP-3) in human synovial fibroblasts. Arthritis Rheum. 1998 Aug;41(8):1398–1406. doi: 10.1002/1529-0131(199808)41:8<1398::AID-ART8>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999 Mar;26(3):717–719. [PubMed] [Google Scholar]
- Brink I., Thiele B., Burmester G. R., Trebeljahr G., Emmrich F., Hiepe F. Effects of anti-CD4 antibodies on the release of IL-6 and TNF-alpha in whole blood samples from patients with systemic lupus erythematosus. Lupus. 1999;8(9):723–730. doi: 10.1191/096120399678840882. [DOI] [PubMed] [Google Scholar]
- Bräuer R., Kette H., Henzgen S., Thoss K. Influence of cyclosporin A on cytokine levels in synovial fluid and serum of rats with antigen-induced arthritis. Agents Actions. 1994 Mar;41(1-2):96–98. doi: 10.1007/BF01986404. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., O'Donnell K., Lawlor K. E., Wicks I. P. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J Clin Invest. 2001 Jun;107(12):1519–1527. doi: 10.1172/JCI12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Q., Londei M. Induction of Th2 cytokines and control of collagen-induced arthritis by nondepleting anti-CD4 Abs. J Immunol. 1996 Sep 15;157(6):2685–2689. [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Fox D. A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997 Apr;40(4):598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Weyand C. M. Interplay of T lymphocytes and HLA-DR molecules in rheumatoid arthritis. Curr Opin Rheumatol. 1993 Mar;5(2):169–177. doi: 10.1097/00002281-199305020-00008. [DOI] [PubMed] [Google Scholar]
- Horneff G., Sack U., Kalden J. R., Emmrich F., Burmester G. R. Reduction of monocyte-macrophage activation markers upon anti-CD4 treatment. Decreased levels of IL-1, IL-6, neopterin and soluble CD14 in patients with rheumatoid arthritis. Clin Exp Immunol. 1993 Feb;91(2):207–213. doi: 10.1111/j.1365-2249.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., Saxne T., van De Loo F. A., Heinegard D., van Den Berg W. B. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999 Nov 1;163(9):5049–5055. [PubMed] [Google Scholar]
- Kassiotis G., Kollias G. TNF and receptors in organ-specific autoimmune disease: multi-layered functioning mirrored in animal models. J Clin Invest. 2001 Jun;107(12):1507–1508. doi: 10.1172/JCI13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R. W., Bräuer R., Stuhlmüller B., Palombo-Kinne E., Burmester G. R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000 Apr 12;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight C. G., Willenbrock F., Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992 Jan 27;296(3):263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- Laan M., Cui Z. H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D. C., Skoogh B. E., Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999 Feb 15;162(4):2347–2352. [PubMed] [Google Scholar]
- Li J. M., Isler P., Dayer J. M., Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995 Apr;84(4):571–576. [PMC free article] [PubMed] [Google Scholar]
- Mason Ursula, Aldrich Jose, Breedveld Ferdinand, Davis Charles B., Elliott Michael, Jackson Mildred, Jorgensen Christian, Keystone Edward, Levy Robert, Tesser John. CD4 coating, but not CD4 depletion, is a predictor of efficacy with primatized monoclonal anti-CD4 treatment of active rheumatoid arthritis. J Rheumatol. 2002 Feb;29(2):220–229. [PubMed] [Google Scholar]
- Mentzel K., Bräuer R. Matrix metalloproteinases, IL-6, and nitric oxide in rat antigen-induced arthritis. Clin Exp Rheumatol. 1998 May-Jun;16(3):269–276. [PubMed] [Google Scholar]
- Moore A. R., Iwamura H., Larbre J. P., Scott D. L., Willoughby D. A. Cartilage degradation by polymorphonuclear leucocytes: in vitro assessment of the pathogenic mechanisms. Ann Rheum Dis. 1993 Jan;52(1):27–31. doi: 10.1136/ard.52.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima S., Saeki Y., Mima T., Sasai M., Nishioka K., Nomura S., Kopf M., Katada Y., Tanaka T., Suemura M. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A. 1998 Jul 7;95(14):8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrí C., Kühnlein P., Buchner E., Schmidt C. B., Franch A., Castell M., Hünig T., Emmrich F., Kinne R. W. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996 Feb;39(2):204–215. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- Petrow P. K., Thoss K., Katenkamp D., Bräuer R. Adoptive transfer of susceptibility to antigen-induced arthritis into severe combined immunodeficient (SCID) mice: role of CD4+ and CD8+ T cells. Immunol Invest. 1996 Jul;25(4):341–353. doi: 10.3109/08820139609059316. [DOI] [PubMed] [Google Scholar]
- Pillinger M. H., Abramson S. B. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am. 1995 Aug;21(3):691–714. [PubMed] [Google Scholar]
- Pohlers D., Nissler K., Frey O., Simon J., Petrow P. K., Kinne R. W., Bräuer R. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen-induced arthritis: influence on T helper cell activation. Clin Exp Immunol. 2004 Mar;135(3):409–415. doi: 10.1111/j.1365-2249.2003.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlers Dirk, Schmidt-Weber Carsten B., Franch Angels, Kuhlmann Jürgen, Bräuer Rolf, Emmrich Frank, Kinne Raimund W. Differential clinical efficacy of anti-CD4 monoclonal antibodies in rat adjuvant arthritis is paralleled by differential influence on NF-kappaB binding activity and TNF-alpha secretion of T cells. Arthritis Res. 2002 Jan 8;4(3):184–189. doi: 10.1186/ar404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryjma J., Mytar B., Loppnow H., Ernst M., Zembala M., Flad H. D. FcR+ and FcR- monocytes differentially secrete monokines during pokeweed mitogen-induced T-cell-monocyte interactions. Immunology. 1992 Feb;75(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- Péterszegi G., Texier S., Robert L. Human helper and memory lymphocytes exhibit an inducible elastin-laminin receptor. Int Arch Allergy Immunol. 1997 Nov;114(3):218–223. doi: 10.1159/000237671. [DOI] [PubMed] [Google Scholar]
- Radsak M., Iking-Konert C., Stegmaier S., Andrassy K., Hänsch G. M. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000 Dec;101(4):521–530. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel C., Sendtner R., Zimmer S., Faist E. Functional analysis of monocyte subsets in surgical sepsis. J Trauma. 1998 May;44(5):743–749. doi: 10.1097/00005373-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H., Lipsky P. E. Anti-CD4 monoclonal antibody therapy in human autoimmune diseases. Curr Dir Autoimmun. 2000;2:24–49. doi: 10.1159/000060506. [DOI] [PubMed] [Google Scholar]
- Simon J., Surber R., Kleinstäuber G., Petrow P. K., Henzgen S., Kinne R. W., Bräuer R. Systemic macrophage activation in locally-induced experimental arthritis. J Autoimmun. 2001 Sep;17(2):127–136. doi: 10.1006/jaut.2001.0534. [DOI] [PubMed] [Google Scholar]
- Veihelmann A., Landes J., Hofbauer A., Dorger M., Refior H. J., Messmer K., Krombach F. Exacerbation of antigen-induced arthritis in inducible nitric oxide synthase-deficient mice. Arthritis Rheum. 2001 Jun;44(6):1420–1427. doi: 10.1002/1529-0131(200106)44:6<1420::AID-ART237>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Marinova-Mutafchieva L., Feldmann M., Maini R. N. Evaluation of TNF-alpha and IL-1 blockade in collagen-induced arthritis and comparison with combined anti-TNF-alpha/anti-CD4 therapy. J Immunol. 2000 Dec 15;165(12):7240–7245. doi: 10.4049/jimmunol.165.12.7240. [DOI] [PubMed] [Google Scholar]
- Yoshino S., Yoshino J. Suppression of chronic antigen-induced arthritis in rats by a monoclonal antibody against the T cell receptor alpha beta. Cell Immunol. 1992 Oct 15;144(2):382–391. doi: 10.1016/0008-8749(92)90253-l. [DOI] [PubMed] [Google Scholar]
- Yui S., Sasaki T., Yamazaki M. Augmentation and suppression of TNF release from macrophages by inflammatory polymorphonuclear leukocytes. Microbiol Immunol. 1993;37(10):801–808. doi: 10.1111/j.1348-0421.1993.tb01708.x. [DOI] [PubMed] [Google Scholar]
- de Hooge A. S., van De Loo F. A., Arntz O. J., van Den Berg W. B. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol. 2000 Dec;157(6):2081–2091. doi: 10.1016/S0002-9440(10)64846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meurs J., van Lent P., Holthuysen A., Lambrou D., Bayne E., Singer I., van den Berg W. Active matrix metalloproteinases are present in cartilage during immune complex-mediated arthritis: a pivotal role for stromelysin-1 in cartilage destruction. J Immunol. 1999 Nov 15;163(10):5633–5639. [PubMed] [Google Scholar]
- van de Loo F. A., Kuiper S., van Enckevort F. H., Arntz O. J., van den Berg W. B. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol. 1997 Jul;151(1):177–191. [PMC free article] [PubMed] [Google Scholar]