Abstract
Objective: To determine whether IL18 has any indirect effects on osteoclastogenesis mediated by T cells in RA synovium, and compare its effects with those of IL1ß and TNFα.
Methods: Resting T cells were isolated from peripheral blood of healthy donors, and stimulated with 2 µg/ml phytohaemagglutinin (PHA) and 0.5 ng/ml IL2 for 24 hours. Synovial T cells were isolated from RA synovial tissue. The levels of soluble receptor activator of the NF-κB ligand (RANKL), osteoprotegerin (OPG), IFNγ, M-CSF, and GM-CSF were determined by ELISA. Membrane bound RANKL expression was analysed by flow cytometry. Commercially available human osteoclast precursors were cocultured with T cells to induce osteoclast formation, which was determined with tartrate resistant acid phosphatase staining and pit formation assay.
Results: In PHA prestimulated T cells or RA synovial T cells, IL18, IL1ß, or TNFα increased soluble RANKL production and membrane bound RANKL expression in a dose dependent manner. IL18, IL1ß, and TNFα did not induce M-CSF, GM-CSF, IFNγ, or OPG production in PHA prestimulated T cells or RA synovial T cells. IL18 increased the number of osteoclasts and bone resorption area on dentine slices in the coculture of human osteoclast precursors with PHA prestimulated T cells or RA synovial T cells; its ability was equivalent to that of IL1ß, but less potent than that of TNFα. In the coculture system, OPG completely blocked osteoclast induction by IL18 or IL1ß, and greatly inhibited induction by TNFα.
Conclusion: IL18, IL1ß, or TNFα can indirectly stimulate osteoclast formation through up regulation of RANKL production from T cells in RA synovitis; IL18 is as effective as IL1ß, but less potent than TNFα.
Full Text
The Full Text of this article is available as a PDF (475.9 KB).
Figure 1.
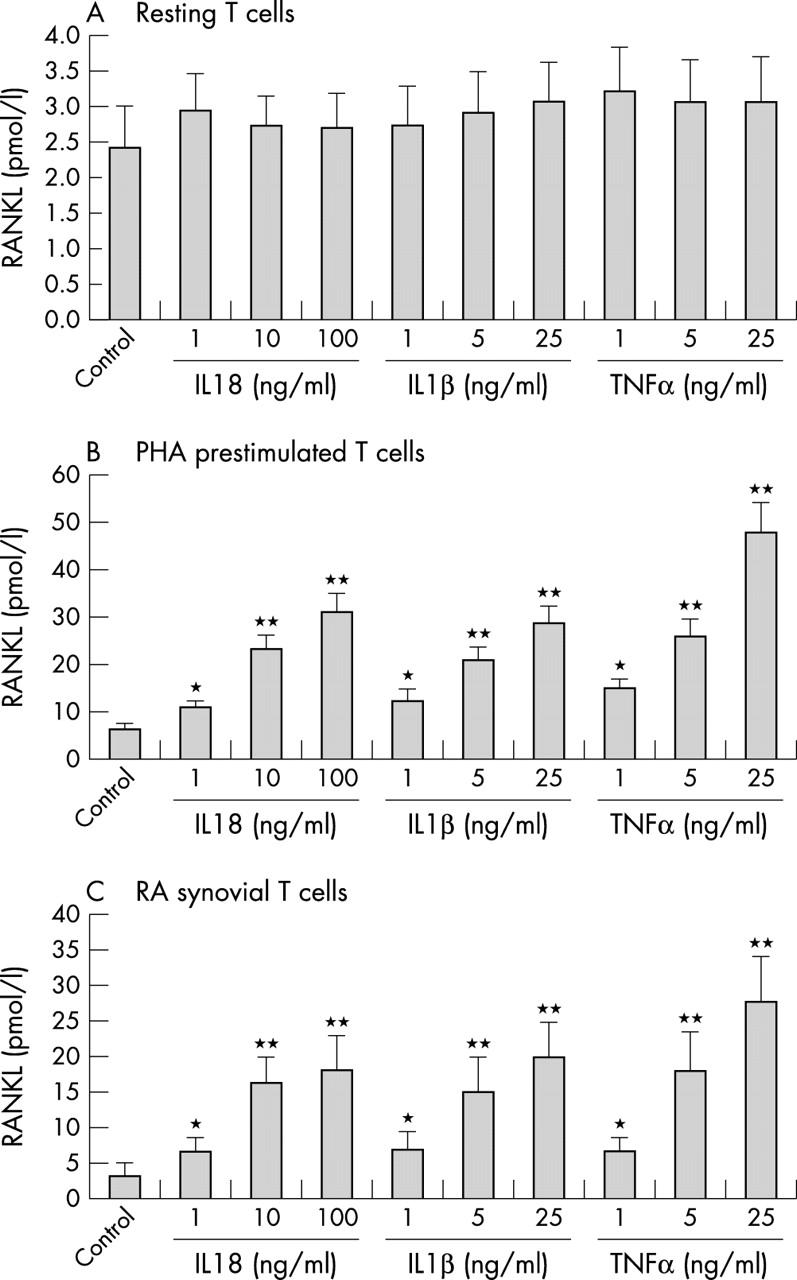
Soluble RANKL production in cultured human T cells in the presence of IL18, IL1ß, or TNFα. (A) T cells were isolated from the peripheral blood of healthy donors. (B) T cells were isolated from the peripheral blood of healthy donors, and then simulated with 2 µg/ml PHA and 0.5 ng/ml IL2 for 24 hours. (C) T cells were isolated from the synovium of patients with RA. Cells were cultured in quadruplicate. The supernatants were harvested after 48 hours' incubation. RANKL levels were determined by ELISA. Data represent mean (SD). *p<0.05, **p<0.01 compared with control group. Results shown are representative of four independent experiments.
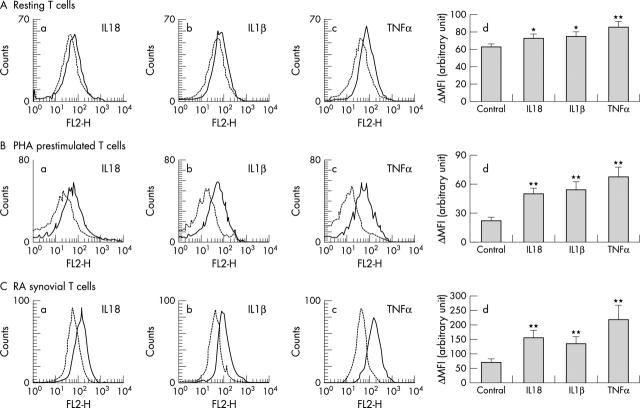
Figure 2.
Membrane bound RANKL expression in cultured human T cells in the presence of IL18, IL1ß, or TNFα. RANKL expression on T cells was analysed by flow cytometry. A representative histogram overlay of 10 ng/ml IL18 (a), 10 ng/ml IL1ß (b), or 10 ng/ml TNFα (c) stimulated (solid line) and unstimulated (broken line) cells. Changes in ΔMFI (difference between the mean fluorescence intensity (MFI) of positive staining and the MFI of the isotype matched negative control) are also shown (d). *p<0.05, **p<0.01 compared with the control group. See fig 1 for other information.
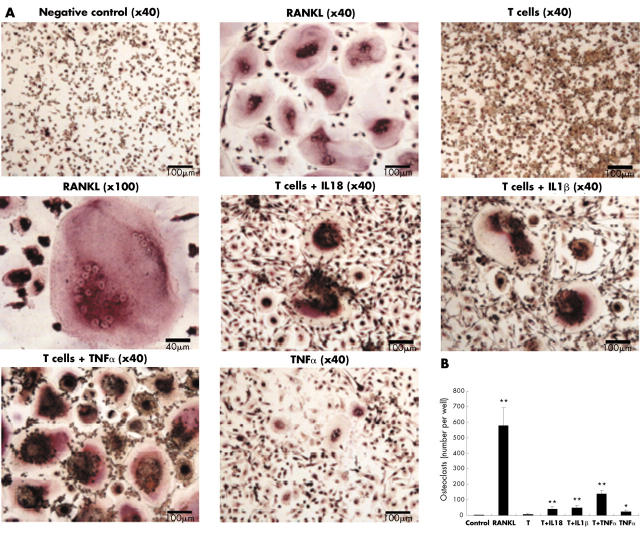
Figure 3.
Osteoclast formation induced from the precursor cells in different conditions. All the cultures were stimulated with 33 ng/ml M-CSF. With the indicated conditions, the cultures were further stimulated with 30 ng/ml soluble RANKL or 10 ng/ml TNFα, or cocultured with PHA prestimulated T cells in the presence of 10 ng/ml IL18, IL1ß, or TNFα. After 7–10 days of culture, cells were fixed and stained for TRAP. TRAP positive giant cells with numerous (⩾3) unstained nuclei were considered as mature osteoclasts. See scale bar in each picture. In (B) T indicates PHA prestimulated T cells. *p<0.05, **p<0.01 compared with the group with T cells only.
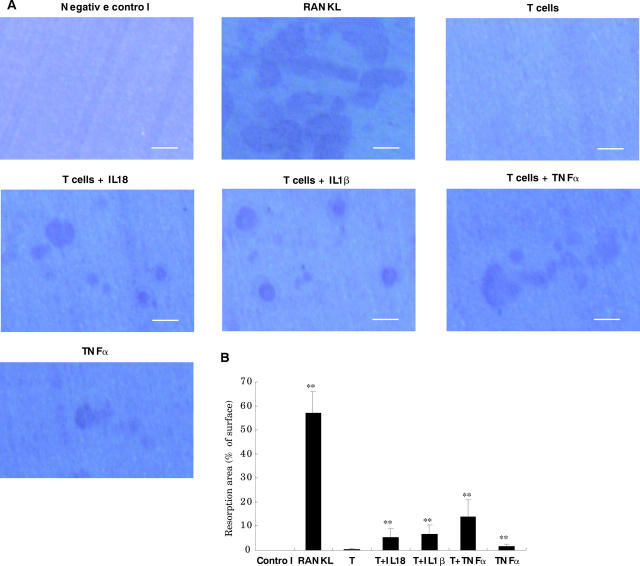
Figure 4.
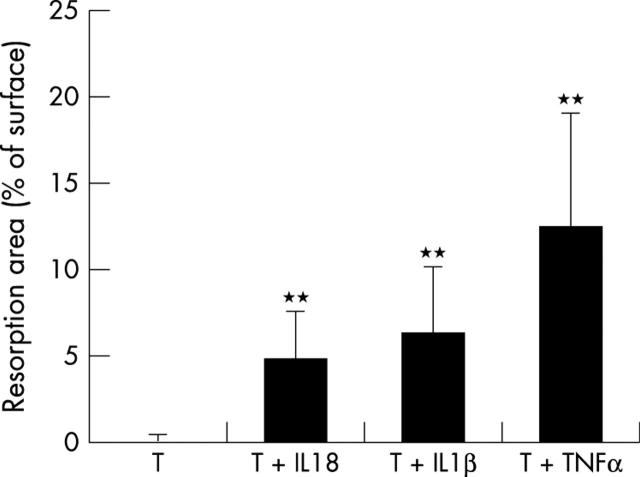
Resorption activity of induced osteoclasts on dentine slices. The osteoclasts were induced as in fig 3. Dentine slices were stained in Mayer's haematoxylin solution, and pits were photographed under a reflected light microscope (A). Scale bar 100 µm. The total pit areas were measured in four randomly selected areas of each dentine slice using an image analysis system (B). In (B) T indicates PHA prestimulated T cells. **p<0.01 compared with the group with T cells only.
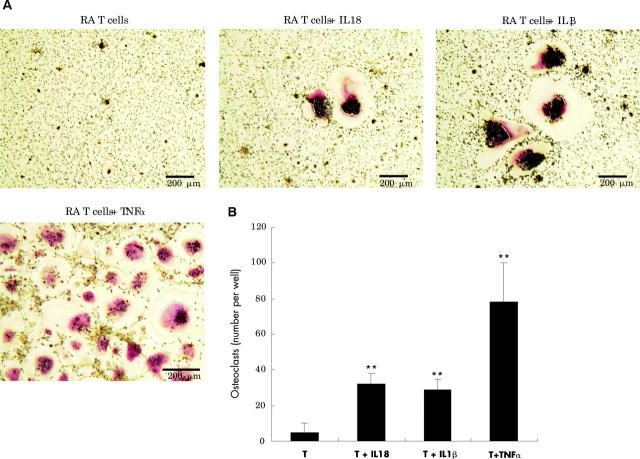
Figure 5.
Osteoclast formation induced from the precursor cells cocultured with RA synovial T cells. All the cultures were stimulated with 33 ng/ml M-CSF. With the indicated conditions, the cultures were further stimulated with 10 ng/ml IL18, IL1ß, or TNFα. Scale bar 200 µm. In (B) T indicates RA synovial T cells. **p<0.01 compared with the group with T cells only.
Figure 6.
Bone resorption activity of osteoclasts induced by RA synovial T cells. The osteoclasts were induced as in fig 5. The total pit areas were measured in four randomly selected areas of each dentine slice using an image analysis system. T indicates RA synovial T cells. **p<0.01 compared with the group with T cells only.
Figure 7.
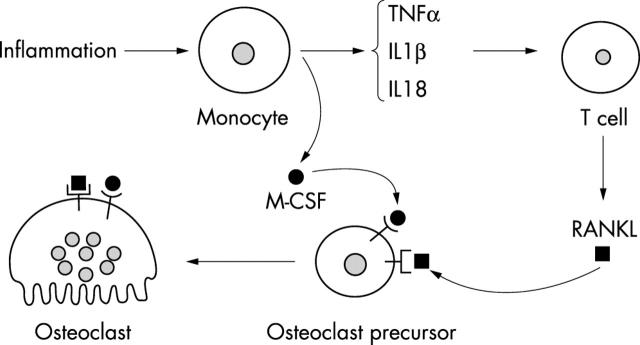
A schematic diagram of IL18, IL1ß, or TNFα indirectly stimulating osteoclastogenesis through T cells in synovitis. In RA synovium, accumulated monocytes/macrophages (and synovial fibroblasts) produce proinflammatory cytokines, such as TNFα, IL1ß, and IL18. These cytokines enhance RANKL production from activated T cells, and then induce osteoclast precursors to differentiate into mature osteoclasts.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Choi Y., Woo K. M., Ko S. H., Lee Y. J., Park S. J., Kim H. M., Kwon B. S. Osteoclastogenesis is enhanced by activated B cells but suppressed by activated CD8(+) T cells. Eur J Immunol. 2001 Jul;31(7):2179–2188. doi: 10.1002/1521-4141(200107)31:7<2179::aid-immu2179>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y., Sabokbar A., Neale S. D., Itonaga I., Torisu T., Athanasou N. A. The effect of macrophage-colony stimulating factor and other humoral factors (interleukin-1, -3, -6, and -11, tumor necrosis factor-alpha, and granulocyte macrophage-colony stimulating factor) on human osteoclast formation from circulating cells. Bone. 2001 Mar;28(3):261–267. doi: 10.1016/s8756-3282(00)00453-1. [DOI] [PubMed] [Google Scholar]
- Gracie J. A., Forsey R. J., Chan W. L., Gilmour A., Leung B. P., Greer M. R., Kennedy K., Carter R., Wei X. Q., Xu D. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999 Nov;104(10):1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Harada Y., Wang J. T., Gorn A. H., Thornhill T. S., Goldring S. R. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998 Apr;152(4):943–951. [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Manning C., Tsay A., Naito A., Pan C., Amento E., Goldring S. R. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000 Feb;43(2):250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Horwood N. J., Kartsogiannis V., Quinn J. M., Romas E., Martin T. J., Gillespie M. T. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999 Nov;265(1):144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- Horwood N. J., Udagawa N., Elliott J., Grail D., Okamura H., Kurimoto M., Dunn A. R., Martin T., Gillespie M. T. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest. 1998 Feb 1;101(3):595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John V., Hock J. M., Short L. L., Glasebrook A. L., Galvin R. J. A role for CD8+ T lymphocytes in osteoclast differentiation in vitro. Endocrinology. 1996 Jun;137(6):2457–2463. doi: 10.1210/endo.137.6.8641199. [DOI] [PubMed] [Google Scholar]
- Jones D. Holstead, Kong Y-Y, Penninger J. M. Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis. 2002 Nov;61 (Suppl 2):ii32–ii39. doi: 10.1136/ard.61.suppl_2.ii32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten L. A., van De Loo F. A., Lubberts E., Helsen M. M., Netea M. G., van Der Meer J. W., Dinarello C. A., van Den Berg W. B. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000 Dec 1;165(11):6553–6558. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- Josien R., Wong B. R., Li H. L., Steinman R. M., Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999 Mar 1;162(5):2562–2568. [PubMed] [Google Scholar]
- Kawashima Masanori, Miossec Pierre. Heterogeneity of response of rheumatoid synovium cell subsets to interleukin-18 in relation to differential interleukin-18 receptor expression. Arthritis Rheum. 2003 Mar;48(3):631–637. doi: 10.1002/art.10825. [DOI] [PubMed] [Google Scholar]
- Kim N., Odgren P. R., Kim D. K., Marks S. C., Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000 Jan 17;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S., Capparelli C., Li J., Elliott R., McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999 Jan 28;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Kotake S., Udagawa N., Hakoda M., Mogi M., Yano K., Tsuda E., Takahashi K., Furuya T., Ishiyama S., Kim K. J. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001 May;44(5):1003–1012. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998 Apr 17;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Leung B. P., McInnes I. B., Esfandiari E., Wei X. Q., Liew F. Y. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000 Jun 15;164(12):6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- Liggett W., Jr, Shevde N., Anklesaria P., Sohoni S., Greenberger J., Glowacki J. Effects of macrophage colony stimulating factor and granulocyte-macrophage colony stimulating factor on osteoclastic differentiation of hematopoietic progenitor cells. Stem Cells. 1993 Sep;11(5):398–411. doi: 10.1002/stem.5530110507. [DOI] [PubMed] [Google Scholar]
- MacDonald B. R., Mundy G. R., Clark S., Wang E. A., Kuehl T. J., Stanley E. R., Roodman G. D. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1986 Apr;1(2):227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Morony S., Capparelli C., Lee R., Shimamoto G., Boone T., Lacey D. L., Dunstan C. R. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1, 25(OH)2D3. J Bone Miner Res. 1999 Sep;14(9):1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- Myint Y. Y., Miyakawa K., Naito M., Shultz L. D., Oike Y., Yamamura K., Takahashi K. Granulocyte/macrophage colony-stimulating factor and interleukin-3 correct osteopetrosis in mice with osteopetrosis mutation. Am J Pathol. 1999 Feb;154(2):553–566. doi: 10.1016/s0002-9440(10)65301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Kobayashi Y., Yamasaki S., Kawakami A., Eguchi K., Sasaki H., Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000 Sep 7;275(3):768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- Ogura T., Ueda H., Hosohara K., Tsuji R., Nagata Y., Kashiwamura S., Okamura H. Interleukin-18 stimulates hematopoietic cytokine and growth factor formation and augments circulating granulocytes in mice. Blood. 2001 Oct 1;98(7):2101–2107. doi: 10.1182/blood.v98.7.2101. [DOI] [PubMed] [Google Scholar]
- Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 Apr 18;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Tominaga K., Yoshimoto T., Torigoe K., Kurimoto M., Matsui K., Hada T., Okamura H., Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000 Feb;12(2):151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- Toraldo Gianluca, Roggia Cristiana, Qian Wei-Ping, Pacifici Roberto, Weitzmann M. Neale. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2002 Dec 18;100(1):125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Horwood N. J., Elliott J., Mackay A., Owens J., Okamura H., Kurimoto M., Chambers T. J., Martin T. J., Gillespie M. T. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997 Mar 17;185(6):1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai T., Hara Y., Kato I. Effects of T cell adoptive transfer into nude mice on alveolar bone resorption induced by endotoxin. J Periodontal Res. 1996 Aug;31(6):414–422. doi: 10.1111/j.1600-0765.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Leung B. P., Arthur H. M., McInnes I. B., Liew F. Y. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001 Jan 1;166(1):517–521. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- Weitzmann M. N., Cenci S., Rifas L., Brown C., Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000 Sep 1;96(5):1873–1878. [PubMed] [Google Scholar]
- Weitzmann M. N., Cenci S., Rifas L., Haug J., Dipersio J., Pacifici R. T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res. 2001 Feb;16(2):328–337. doi: 10.1359/jbmr.2001.16.2.328. [DOI] [PubMed] [Google Scholar]
- Wong B. R., Rho J., Arron J., Robinson E., Orlinick J., Chao M., Kalachikov S., Cayani E., Bartlett F. S., 3rd, Frankel W. N. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997 Oct 3;272(40):25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Takeda K., Tanaka T., Ohkusu K., Kashiwamura S., Okamura H., Akira S., Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998 Oct 1;161(7):3400–3407. [PubMed] [Google Scholar]