Abstract
Objective: To measure synovial tissue interleukin-18 (IL-18) expression in patients with inflammatory arthritis, and to identify associations with serum levels, disease activity, and response to treatment.
Methods: Synovial tissue biopsies and serum samples were obtained from patients with early, active, rheumatoid arthritis (RA) (n = 12), undifferentiated seronegative arthritis (SnA) (n = 9), psoriatic arthritis (PsA) (n = 5), and reactive arthritis (ReA) (n = 2) before and one year after introduction of disease modifying antirheumatic drug (DMARD) treatment. Osteoarthritis (OA) tissues were compared. Tissue IL-18 expression was determined after immunohistochemical staining using a semiquantitative scale. Serum IL-18 was measured by enzyme linked immunosorbent assay.
Results: Before treatment was started, tissue IL-18 expression was increased in each diagnostic group compared with OA (p<0.05). Tissue IL-18 expression was correlated with serum C reactive protein levels (r = 0.53, p = 0.003) but not with serum IL-18. After DMARD treatment, 12 patients (five RA, four SnA, three PsA) were re-evaluated. Decreases in tissue IL-18 expression were observed in eight, although the trend did not reach significance (p = 0.068). Changes in tissue IL-18 expression were correlated with changes in serum IL-18 (r = 0.62, p = 0.041) and C reactive protein (r = 0.72, p = 0.009).
Conclusions: Synovial tissue IL-18 expression was correlated with disease activity in inflammatory arthritis. After treatment, tissue levels changed in parallel with changes in serum IL-18 and with changes in the acute phase response. These observations support a role for IL-18 in the pathophysiology of inflammatory arthritis.
Full Text
The Full Text of this article is available as a PDF (163.6 KB).
Figure 1.
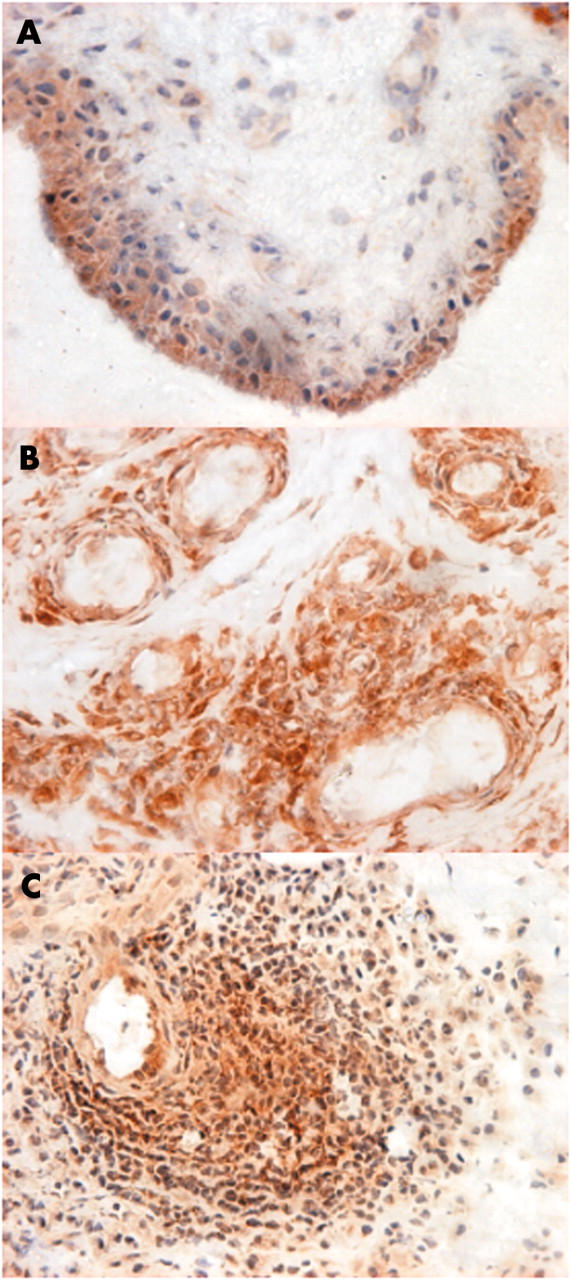
Photomicrographs of synovial tissue sections obtained from patients with active inflammatory arthritis, showing interleukin-18 (IL-18) expression in the lining layer (A), endothelial cells (B), and aggregating inflammatory cells in the sublining layer (C). IL-18 positive cells are shown actively infiltrating perivascular tissue in panels B and C. (Original magnification x200.)
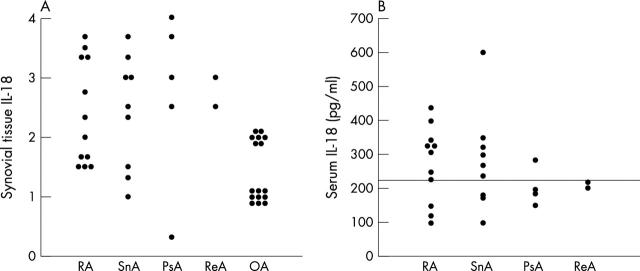
Figure 2.
Interleukin-18 (IL-18) expression in synovial tissue (A) and serum (B) in patients with active inflammatory arthritis. After immunohistochemical staining, tissue IL-18 expression was evaluated using a semiquantitative scale of 0 to 4, where 0 represents little expression and 4 intense expression. Serum IL-18 was measured by enzyme linked immunosorbent assay. The upper limit of the normal reference range for serum IL-18 in healthy subjects is indicated. OA, osteoarthritis; PsA, psoriatic arthritis; RA, rheumatoid arthritis; ReA, reactive arthritis; SnA, seronegative arthritis.
Figure 3.
Synovial tissue interleukin-18 (IL-18) expression and serum C reactive protein in patients with active inflammatory arthritis. The correlation coefficient was calculated using Spearman's rank correlation test.
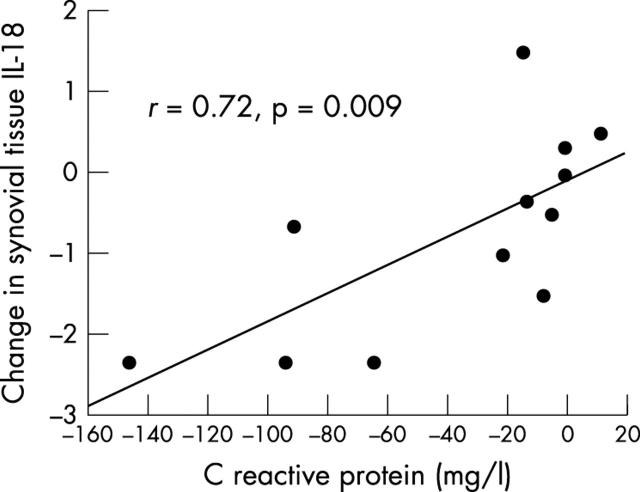
Figure 4.
Changes in synovial tissue interleukin-18 (IL-18) expression versus changes in serum C reactive protein levels after one year of methotrexate (n = 7) or sulphasalazine (n = 5) treatment in patients with inflammatory arthritis. The correlation coefficient was calculated using Spearman's rank correlation test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor B., Dougados M., Mijiyawa M. Critères de classification des spondylarthropathies. Rev Rhum Mal Osteoartic. 1990 Feb;57(2):85–89. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Roux-Lombard P., Murphy E., Kane D., FitzGerald O., Dayer J-M. Serum interleukin 18 and interleukin 18 binding protein in rheumatoid arthritis. Ann Rheum Dis. 2002 Aug;61(8):726–729. doi: 10.1136/ard.61.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Novick D., Puren A. J., Fantuzzi G., Shapiro L., Mühl H., Yoon D. Y., Reznikov L. L., Kim S. H., Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998 Jun;63(6):658–664. [PubMed] [Google Scholar]
- Dolhain R. J., Tak P. P., Dijkmans B. A., De Kuiper P., Breedveld F. C., Miltenburg A. M. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol. 1998 May;37(5):502–508. doi: 10.1093/rheumatology/37.5.502. [DOI] [PubMed] [Google Scholar]
- Gracie J. A., Forsey R. J., Chan W. L., Gilmour A., Leung B. P., Greer M. R., Kennedy K., Carter R., Wei X. Q., Xu D. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999 Nov;104(10):1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Kuida K., Tsutsui H., Ku G., Hsiao K., Fleming M. A., Hayashi N., Higashino K., Okamura H., Nakanishi K. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997 Jan 10;275(5297):206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., van De Loo F. A., Lubberts E., Helsen M. M., Netea M. G., van Der Meer J. W., Dinarello C. A., van Den Berg W. B. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J Immunol. 2000 Dec 1;165(11):6553–6558. doi: 10.4049/jimmunol.165.11.6553. [DOI] [PubMed] [Google Scholar]
- Joosten Leo A. B., Radstake Timothy R. D., Lubberts Erik, van den Bersselaar Liduine A. M., van Riel Piet L. C. M., van Lent Peter L. E. M., Barrera Pilar, van den Berg Wim B. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003 Feb;48(2):339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- Kaser A., Novick D., Rubinstein M., Siegmund B., Enrich B., Koch R. O., Vogel W., Kim S. H., Dinarello C. A., Tilg H. Interferon-alpha induces interleukin-18 binding protein in chronic hepatitis C patients. Clin Exp Immunol. 2002 Aug;129(2):332–338. doi: 10.1046/j.1365-2249.2002.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Terajima H., Harigai M., Hara M., Kamatani N. Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset Still's disease. Arthritis Rheum. 2001 Jul;44(7):1716–1717. doi: 10.1002/1529-0131(200107)44:7<1716::AID-ART298>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Yamamura M., Taniai M., Yamauchi H., Tanimoto T., Kurimoto M., Miyawaki S., Amano T., Takeuchi T., Makino H. Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset Still's disease. Arthritis Rheum. 2001 Mar;44(3):550–560. doi: 10.1002/1529-0131(200103)44:3<550::AID-ANR103>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kawashima Masanori, Miossec Pierre. Heterogeneity of response of rheumatoid synovium cell subsets to interleukin-18 in relation to differential interleukin-18 receptor expression. Arthritis Rheum. 2003 Mar;48(3):631–637. doi: 10.1002/art.10825. [DOI] [PubMed] [Google Scholar]
- Leung B. P., McInnes I. B., Esfandiari E., Wei X. Q., Liew F. Y. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000 Jun 15;164(12):6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- Ludwiczek Othmar, Kaser Arthur, Novick Daniela, Dinarello Charles A., Rubinstein Menachem, Vogel Wolfgang, Tilg Herbert. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J Clin Immunol. 2002 Nov;22(6):331–337. doi: 10.1023/a:1020600230977. [DOI] [PubMed] [Google Scholar]
- McInnes I. B., Gracie J. A., Leung B. P., Wei X. Q., Liew F. Y. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000 Jul;21(7):312–315. doi: 10.1016/s0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- Morel J. C., Park C. C., Kumar P., Koch A. E. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001 Oct;81(10):1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996 Jan;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Möller B., Kukoc-Zivojnov N., Kessler U., Rehart S., Kaltwasser J. P., Hoelzer D., Kalina U., Ottmann O. G. Expression of interleukin-18 and its monokine-directed function in rheumatoid arthritis. Rheumatology (Oxford) 2001 Mar;40(3):302–309. doi: 10.1093/rheumatology/40.3.302. [DOI] [PubMed] [Google Scholar]
- Möller B., Paulukat J., Nold M., Behrens M., Kukoc-Zivojnov N., Kaltwasser J. P., Pfeilschifter J., Mühl H. Interferon-gamma induces expression of interleukin-18 binding protein in fibroblast-like synoviocytes. Rheumatology (Oxford) 2003 Mar;42(3):442–445. doi: 10.1093/rheumatology/keg146. [DOI] [PubMed] [Google Scholar]
- Novick D., Kim S. H., Fantuzzi G., Reznikov L. L., Dinarello C. A., Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999 Jan;10(1):127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Okamura H., Tsutsi H., Komatsu T., Yutsudo M., Hakura A., Tanimoto T., Torigoe K., Okura T., Nukada Y., Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995 Nov 2;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Park C. C., Morel J. C., Amin M. A., Connors M. A., Harlow L. A., Koch A. E. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001 Aug 1;167(3):1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- Pittoni V., Bombardieri M., Spinelli F. R., Scrivo R., Alessandri C., Conti F., Spadaro A., Valesini G. Anti-tumour necrosis factor (TNF) alpha treatment of rheumatoid arthritis (infliximab) selectively down regulates the production of interleukin (IL) 18 but not of IL12 and IL13. Ann Rheum Dis. 2002 Aug;61(8):723–725. doi: 10.1136/ard.61.8.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C., Joosten L. A., Helsen M. M., Sattonnet-Roche P., Siegfried C., Alouani S., van De Loo F. A., Graber P., Aloni S., Cirillo R. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001 Dec;108(12):1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puren A. J., Fantuzzi G., Gu Y., Su M. S., Dinarello C. A. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998 Feb 1;101(3):711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M., Whelan A., Feighery C., Bresnihan B. Changes in lymphocyte infiltration of the synovial membrane and the clinical course of rheumatoid arthritis. Arthritis Rheum. 1989 Apr;32(4):361–369. doi: 10.1002/anr.1780320402. [DOI] [PubMed] [Google Scholar]
- Roux-Lombard P., Steiner G. Preliminary report on cytokine determination in human synovial fluids: a consensus study of the European Workshop for Rheumatology Research. The Cytokine Consensus Study Group of the European Workshop for Rheumatology Research. Clin Exp Rheumatol. 1992 Sep-Oct;10(5):515–520. [PubMed] [Google Scholar]
- Tak P. P., Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000 Dec;43(12):2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Kummer J. A., Hack C. E., Daha M. R., Smeets T. J., Erkelens G. W., Meinders A. E., Kluin P. M., Breedveld F. C. Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994 Dec;37(12):1735–1743. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Harigai M., Kawaguchi Y., Ohta S., Sugiura T., Takagi K., Ohsako-Higami S., Fukasawa C., Hara M., Kamatani N. Mature form of interleukin 18 is expressed in rheumatoid arthritis synovial tissue and contributes to interferon-gamma production by synovial T cells. J Rheumatol. 2001 Aug;28(8):1779–1787. [PubMed] [Google Scholar]
- Taniguchi M., Nagaoka K., Kunikata T., Kayano T., Yamauchi H., Nakamura S., Ikeda M., Orita K., Kurimoto M. Characterization of anti-human interleukin-18 (IL-18)/interferon-gamma-inducing factor (IGIF) monoclonal antibodies and their application in the measurement of human IL-18 by ELISA. J Immunol Methods. 1997 Aug 7;206(1-2):107–113. doi: 10.1016/s0022-1759(97)00094-x. [DOI] [PubMed] [Google Scholar]
- Torigoe K., Ushio S., Okura T., Kobayashi S., Taniai M., Kunikata T., Murakami T., Sanou O., Kojima H., Fujii M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997 Oct 10;272(41):25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- Veale D., Rogers S., Fitzgerald O. Classification of clinical subsets in psoriatic arthritis. Br J Rheumatol. 1994 Feb;33(2):133–138. doi: 10.1093/rheumatology/33.2.133. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L. Cytokine measurements and interpretation of cytokine assays in human disease. J Clin Immunol. 1994 Nov;14(6):327–339. doi: 10.1007/BF01546317. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Kawashima M., Taniai M., Yamauchi H., Tanimoto T., Kurimoto M., Morita Y., Ohmoto Y., Makino H. Interferon-gamma-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum. 2001 Feb;44(2):275–285. doi: 10.1002/1529-0131(200102)44:2<275::AID-ANR44>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Yanni G., Nabil M., Farahat M. R., Poston R. N., Panayi G. S. Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann Rheum Dis. 1994 May;53(5):315–322. doi: 10.1136/ard.53.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef P. P., Haynes D. R., Triantafillou S., Parker A., Gamble J. R., Roberts-Thomson P. J., Ahern M. J., Smith M. D. Effects of pulse methylprednisolone on inflammatory mediators in peripheral blood, synovial fluid, and synovial membrane in rheumatoid arthritis. Arthritis Rheum. 1997 Aug;40(8):1400–1408. doi: 10.1002/art.1780400807. [DOI] [PubMed] [Google Scholar]