Abstract
Objective: To correlate the number of chondrocytes in healthy and osteoarthritic human articular cartilage with age, and to evaluate the influence of donor age on total proteoglycan synthesis.
Methods: Chondrocytes were isolated from human articular cartilage derived from hip joints with and without osteoarthritic lesions. The cell number was normalised to cartilage sample wet weight. In addition, the influence of age on chondrocyte numbers was assessed histomorphometrically. Chondrocytes were grown as monolayer cultures for seven days in a chemically defined serum-free basal medium. Total proteoglycan synthesis was measured by [35S]sulphate incorporation into newly synthesised macromolecules.
Results: Chondrocyte numbers in healthy cartilage decreased significantly with advancing age (r = –0.69, p<0.0001). In contrast to healthy specimens, chondrocyte numbers were decreased in osteoarthritic cartilage irrespective of and unrelated to age, and differed markedly, by an average of 38%, from the cell numbers found in healthy individuals (p<0.0001). Regarding synthesis of matrix macromolecules, no dependence on patients' age, either in healthy or in osteoarthritic specimens, could be observed.
Conclusions: Under the experimental conditions employed, chondrocytes from healthy and osteoarthritic joints synthesised comparable amounts of cartilage macromolecules, independent of age or underlying osteoarthritic disease. Thus the decrease in chondrocyte number in aging and osteoarthritic joints could be a crucial factor in limiting tissue replenishment.
Full Text
The Full Text of this article is available as a PDF (82.8 KB).
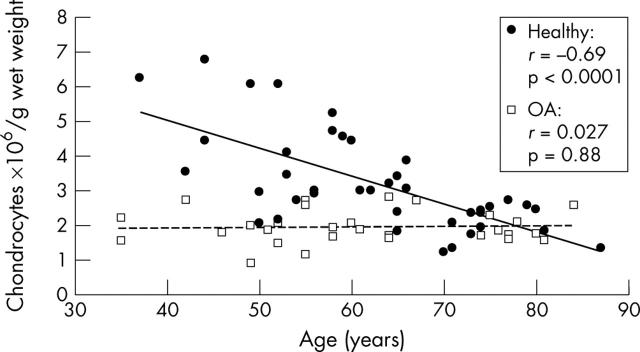
Figure 1.
Changes in the total chondrocyte numbers of healthy and osteoarthritic articular cartilage with increasing age. Chondrocytes were enzymatically released from their extracellular matrix in 0.2% collagenase B. Directly after digestion cell numbers were assessed using light microscopy. The values are given as chondrocytes x106/g wet weight. There was a decrease in chondrocyte numbers in healthy articular cartilage (black circles, black regression line) as a function of age (n = 41; r = –0.69, p<0.0001). Cellularity in osteoarthritic cartilage (white squares, dashed regression line) showed a significant reduction in cell number compared with healthy cartilage (p<0.0001), but no dependence on patients' age (n = 30; r = 0.027, p = 0.88).
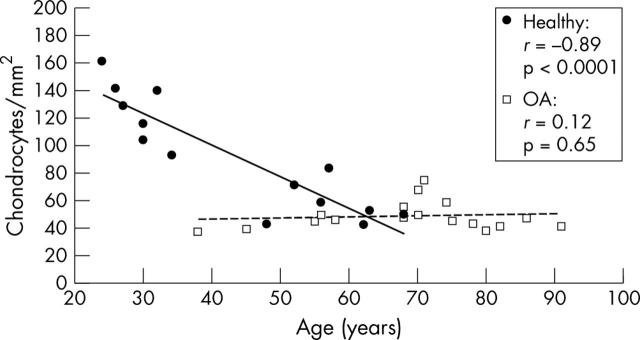
Figure 2.
Chondrocyte numbers in histological sections from healthy and osteoarthritic cartilage. The values are given as chondrocytes/mm2 cartilage. Chondrocyte numbers in healthy articular cartilage (black circles, black regression line) decrease as a function of age (n = 14; r = –0.89, p<0.0001). In osteoarthritic cartilage (white squares, dashed regression line) no reduction in cell number could be seen (n = 17; r = 0.12, p<0.65). Analysis of the healthy v the osteoarthritic group showed a significant difference in cell numbers: healthy group, 91.6 (10.9) cells/mm2, v osteoarthritis group, 48.6 (2.5) cells/mm2 (mean (SEM)), p<0.007.
Figure 3.
(A) Healthy (black circles, black regression line) and osteoarthritic (white squares, dashed regression line) human articular chondrocytes do not lose their biosynthetic capacity with age. Cells were cultured in serum-free basal medium without the addition of growth factors for seven days. The rate of proteoglycan synthesis was measured by [35S]sulphate incorporation into newly synthesised matrix proteoglycans present in the cell layer. Values were normalised to protein content and are given as counts per min/mg protein (cpm/mg protein). No age dependent decrease in total proteoglycan synthesis could be shown for healthy chondrocytes (r = –0.23, p = 0.38) or osteoarthritic chondrocytes (r = –0.19, p = 0.55). (B) Proteoglycan synthesis of postnatal human articular chondrocytes. Chondrocytes derived from healthy patients <55 years (white bars, n = 8), >55 years (light grey bars, n = 8), and osteoarthritis patients (dark grey bars, n = 13) were incubated in serum-free basal medium for seven days. On the final day of the incubation period, cell cultures were labelled with [35S]sulphate for six hours. The incorporated radiolabel in newly synthesised matrix macromolecules present in the cell layer was then measured, normalised to protein content, and given as cpm/mg protein. Values are mean (SEM). No difference between these groups could be determined (NS).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Osborne D., Woodhouse S., Davidson C. Sulfation of chondroitin sulfate in human articular cartilage. The effect of age, topographical position, and zone of cartilage on tissue composition. J Biol Chem. 1999 May 28;274(22):15892–15900. doi: 10.1074/jbc.274.22.15892. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Guitian R., Vázquez-Martul E., de Toro F. J., Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998 Feb;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bobacz K., Gruber R., Soleiman A., Erlacher L., Smolen J. S., Graninger W. B. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003 Sep;48(9):2501–2508. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- Bobacz K., Gruber R., Soleiman A., Graninger W. B., Luyten F. P., Erlacher L. Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthritis Cartilage. 2002 May;10(5):394–401. doi: 10.1053/joca.2002.0522. [DOI] [PubMed] [Google Scholar]
- Brocklehurst R., Bayliss M. T., Maroudas A., Coysh H. L., Freeman M. A., Revell P. A., Ali S. Y. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am. 1984 Jan;66(1):95–106. [PubMed] [Google Scholar]
- Buckwalter J. A., Rosenberg L. C. Electron microscopic studies of cartilage proteoglycans. Direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J Biol Chem. 1982 Aug 25;257(16):9830–9839. [PubMed] [Google Scholar]
- Buckwalter J. A., Roughley P. J., Rosenberg L. C. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994 Aug 1;28(5):398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Woo S. L., Goldberg V. M., Hadley E. C., Booth F., Oegema T. R., Eyre D. R. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993 Oct;75(10):1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Woo S. L., Goldberg V. M., Hadley E. C., Booth F., Oegema T. R., Eyre D. R. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993 Oct;75(10):1533–1548. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- Creamer P., Hochberg M. C. Osteoarthritis. Lancet. 1997 Aug 16;350(9076):503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Ettinger W. H., Neuhaus J. M., Mallon K. P. Knee osteoarthritis and physical functioning: evidence from the NHANES I Epidemiologic Followup Study. J Rheumatol. 1991 Apr;18(4):591–598. [PubMed] [Google Scholar]
- DeGroot J., Verzijl N., Bank R. A., Lafeber F. P., Bijlsma J. W., TeKoppele J. M. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999 May;42(5):1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Erlacher L., McCartney J., Piek E., ten Dijke P., Yanagishita M., Oppermann H., Luyten F. P. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. J Bone Miner Res. 1998 Mar;13(3):383–392. doi: 10.1359/jbmr.1998.13.3.383. [DOI] [PubMed] [Google Scholar]
- Felson D. T. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990 Dec;20(3 Suppl 1):42–50. doi: 10.1016/0049-0172(90)90046-i. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Blanco F., Kaelin A., Desgeorges A., Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995 Jul;38(7):960–968. doi: 10.1002/art.1780380712. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Aging and osteoarthritis: basic mechanisms. J Am Geriatr Soc. 1993 Jul;41(7):760–770. doi: 10.1111/j.1532-5415.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]
- Hardingham T., Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990 Dec;20(3 Suppl 1):12–33. doi: 10.1016/0049-0172(90)90044-g. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Ochs R. L., Komiya S., Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998 Sep;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Huch K. Knee and ankle: human joints with different susceptibility to osteoarthritis reveal different cartilage cellularity and matrix synthesis in vitro. Arch Orthop Trauma Surg. 2001 Jun;121(6):301–306. doi: 10.1007/s004020000225. [DOI] [PubMed] [Google Scholar]
- Kempson G. E. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991 Oct 31;1075(3):223–230. doi: 10.1016/0304-4165(91)90270-q. [DOI] [PubMed] [Google Scholar]
- Lafeber F. P., Vander Kraan P. M., Huber-Bruning O., Vanden Berg W. B., Bijlsma J. W. Osteoarthritic human cartilage is more sensitive to transforming growth factor beta than is normal cartilage. Br J Rheumatol. 1993 Apr;32(4):281–286. doi: 10.1093/rheumatology/32.4.281. [DOI] [PubMed] [Google Scholar]
- Luyten F. P., Yu Y. M., Yanagishita M., Vukicevic S., Hammonds R. G., Reddi A. H. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992 Feb 25;267(6):3691–3695. [PubMed] [Google Scholar]
- MEACHIM G., COLLINS D. H. Cell counts of normal and osteoarthritic articular cartilage in relation to the uptake of sulphate (35SO4) in vitro. Ann Rheum Dis. 1962 Mar;21:45–50. doi: 10.1136/ard.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILES J. S., EICHELBERGER L. BIOCHEMICAL STUDIES OF HUMAN CARTILAGE DURING THE AGING PROCESS. J Am Geriatr Soc. 1964 Jan;12:1–20. doi: 10.1111/j.1532-5415.1964.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Maroudas A., Ziv I., Weisman N., Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22(2):159–169. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- Mitrovic D., Quintero M., Stankovic A., Ryckewaert A. Cell density of adult human femoral condylar articular cartilage. Joints with normal and fibrillated surfaces. Lab Invest. 1983 Sep;49(3):309–316. [PubMed] [Google Scholar]
- Muehleman C., Chubinskaya S., Cole A. A., Noskina Y., Arsenis C., Kuettner K. E. 1997 William J. Stickel Gold Award. Morphological and biochemical properties of metatarsophalangeal joint cartilage. J Am Podiatr Med Assoc. 1997 Oct;87(10):447–459. doi: 10.7547/87507315-87-10-447. [DOI] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Peyron J. G. Risk factors in osteoarthritis--how do they work? J Rheumatol. 1987 May;14(Spec No):1–2. [PubMed] [Google Scholar]
- Quintero M., Mitrovic D. R., Stankovic A., de Sèze S., Miravet L., Ryckewaert A. Aspects cellulaires du vieillissement du cartilage articulaire. I. Cartilage condylien à surface normale, prèlevè dans les genoux normaux. Rev Rhum Mal Osteoartic. 1984 Jul-Sep;51(7-8):375–379. [PubMed] [Google Scholar]
- Quintero M., Mitrovic D. R., Stankovic M. A., de Sèze S., Miravet L., Ryckewaert A. Aspects cellulaires du vieillissement du cartilage articulaire. II. Cartilage condylien à surface fissurée prélevé dans les genoux "normaux" et "arthrosiques". Rev Rhum Mal Osteoartic. 1984 Oct;51(9):445–449. [PubMed] [Google Scholar]
- Schafer S. J., Luyten F. P., Yanagishita M., Reddi A. H. Proteoglycan metabolism is age related and modulated by isoforms of platelet-derived growth factor in bovine articular cartilage explant cultures. Arch Biochem Biophys. 1993 May;302(2):431–438. doi: 10.1006/abbi.1993.1236. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A. The cell density of human articular and costal cartilage. J Anat. 1967 Sep;101(Pt 4):753–763. [PMC free article] [PubMed] [Google Scholar]
- Venn M. F. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis. 1978 Apr;37(2):168–174. doi: 10.1136/ard.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen G., Cornelissen M., Almqvist K. F., Wang L., Elewaut D., Broddelez C., de Ridder L., Veys E. M. Influence of aging on the synthesis and morphology of the aggrecans synthesized by differentiated human articular chondrocytes. Osteoarthritis Cartilage. 2000 May;8(3):170–179. doi: 10.1053/joca.1999.0287. [DOI] [PubMed] [Google Scholar]
- Vignon E., Arlot M., Patricot L. M., Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res. 1976 Nov-Dec;(121):303–308. [PubMed] [Google Scholar]
- Weightman B. Tensile fatigue of human articular cartilage. J Biomech. 1976;9(4):193–200. doi: 10.1016/0021-9290(76)90004-x. [DOI] [PubMed] [Google Scholar]