Abstract
Objective: To describe a registry set up to monitor children treated with etanercept in Germany and Austria.
Methods: Giannini's criteria, duration of morning stiffness, number of swollen, tender and contracted joints, adverse events, and reasons for discontinuation were assessed.
Results: 322 patients with juvenile idiopathic arthritis (JIA) and 12 additional patients with non-JIA rheumatic diagnoses were included. Therapeutic efficacy was observed from one month after treatment was started. The number of patients with significant improvement and the degree of improvement increased during the first year. The mean (SD) number of tender and swollen joints decreased from 9 (9) and 8.4 (9) to 3.0 (6.5) and 4.5 (7) after one month, and to 2.2 (5.5) and 3.3 (5.5) after three months; morning stiffness decreased from 45 (65) minutes to 12 (30) and 7 (19) after one and three months (p<0.001 for all). Using Gianinni's criteria of 30%, 50%, and 70% improvement, a therapeutic response in JIA patients was achieved in, respectively, 66%, 54%, and 30% after one month, 78%, 61%, and 38% after three months, and 83%, 72%, and 52% after six months. Therapeutic efficacy was lower in patients with systemic onset arthritis. Overall tolerability was good: in 592 patient treatment-years there were 69 reports of adverse events in 56 patients, including one CNS demyelination. There were no opportunistic infections or lupus-like reactions. Treatment was discontinued in 53 JIA patients, in 25 because of lack of efficacy.
Conclusion: Etanercept treatment was safe and led to a significant improvement in most JIA patients resistant to conventional treatment.
Full Text
The Full Text of this article is available as a PDF (89.7 KB).
Figure 1.
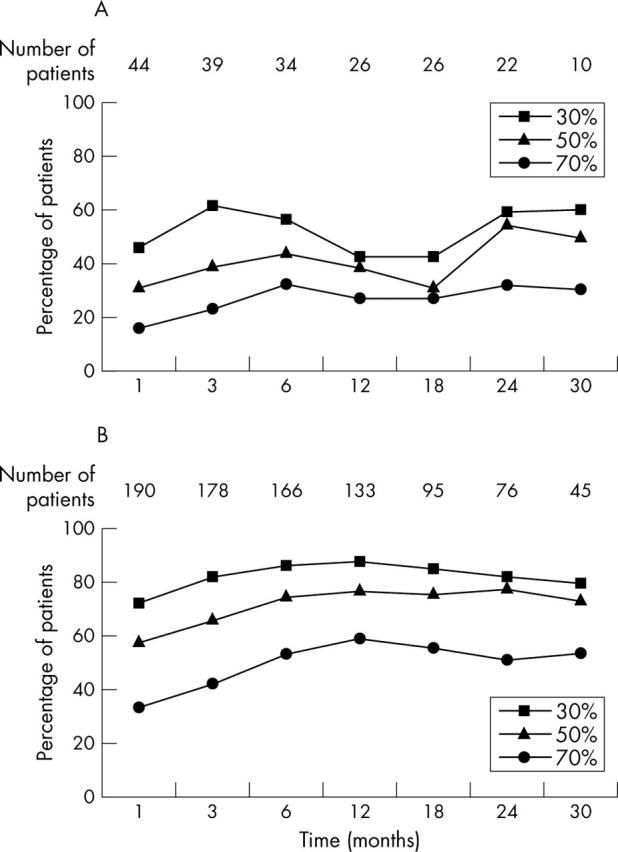
Incidence of 30%, 50%, and 70% improvement in patients with juvenile idiopathic arthritis (JIA) receiving etanercept treatment, according to the core set criteria. Data on 275 patients are included. Analysis was undertaken by LCOF (last observation carried forward). (A) 48 JIA patients with systemic onset and a treatment duration of at least two months. Four patients were excluded because treatment had been discontinued earlier; in eight patients month 1 data were missing; eight patients with systemic onset JIA discontinued treatment prematurely. At last report, a 30%, 50%, or 70% response level was reported in one, one, and two patients, respectively, while four were non-responders. (B) 222 JIA patients with non-systemic-onset. In 32 patients month 1 data were missing. The number of non-systemic-onset JIA patients who met the 30%, 50% and 70% response criteria exceeded the number of systemic onset JIA patients who met the core set criteria. Twenty four of 222 patients with non-systemic-onset JIA discontinued prematurely. At last report a 30%, 50%, or 70% response level was reported in four, six, and eight patients, while only six were non-responders.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer E. J., Jr, Bass J., Baum J., Cassidy J. T., Fink C., Jacobs J., Hanson V., Levinson J. E., Schaller J., Stillman J. S. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977 Mar;20(2 Suppl):195–199. [PubMed] [Google Scholar]
- Giannini E. H., Ruperto N., Ravelli A., Lovell D. J., Felson D. T., Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997 Jul;40(7):1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneff G., Forster J., Seyberth H. W., Michels H., Arbeitgemeinschaft Kinder- und Jugendrheumatologie Empfehlungen der Arbeitsgemeinschaft Kinder- und Jugendrheumatologie zur Therapie mit Etanercept (P75 TNF-alpha-Rezeptor-Immunglobulinfusionsprotein). Kommission Pharmakotherapie. Z Rheumatol. 2000 Dec;59(6):365–369. doi: 10.1007/s003930070043. [DOI] [PubMed] [Google Scholar]
- Kietz D. A., Pepmueller P. H., Moore T. L. Clinical response to etanercept in polyarticular course juvenile rheumatoid arthritis. J Rheumatol. 2001 Feb;28(2):360–362. [PubMed] [Google Scholar]
- Kietz D. A., Pepmueller P. H., Moore T. L. Therapeutic use of etanercept in polyarticular course juvenile idiopathic arthritis over a two year period. Ann Rheum Dis. 2002 Feb;61(2):171–173. doi: 10.1136/ard.61.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdenne P., Vähäsalo P., Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003 Mar;62(3):245–247. doi: 10.1136/ard.62.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell D. J., Giannini E. H., Reiff A., Cawkwell G. D., Silverman E. D., Nocton J. J., Stein L. D., Gedalia A., Ilowite N. T., Wallace C. A. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000 Mar 16;342(11):763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- Lovell Daniel J., Giannini Edward H., Reiff Andreas, Jones Olcay Y., Schneider Rayfel, Olson Judyann C., Stein Leonard D., Gedalia Abraham, Ilowite Norman T., Wallace Carol A. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis: interim results from an ongoing multicenter, open-label, extended-treatment trial. Arthritis Rheum. 2003 Jan;48(1):218–226. doi: 10.1002/art.10710. [DOI] [PubMed] [Google Scholar]
- Minden K., Kiessling U., Listing J., Niewerth M., Döring E., Meincke J., Schöntube M., Zink A. Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol. 2000 Sep;27(9):2256–2263. [PubMed] [Google Scholar]
- Mohan N., Edwards E. T., Cupps T. R., Oliverio P. J., Sandberg G., Crayton H., Richert J. R., Siegel J. N. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001 Dec;44(12):2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Petty R. E. Prognosis in children with rheumatic diseases: justification for consideration of new therapies. Rheumatology (Oxford) 1999 Aug;38(8):739–742. doi: 10.1093/rheumatology/38.8.739. [DOI] [PubMed] [Google Scholar]
- Petty R. E., Southwood T. R., Baum J., Bhettay E., Glass D. N., Manners P., Maldonado-Cocco J., Suarez-Almazor M., Orozco-Alcala J., Prieur A. M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998 Oct;25(10):1991–1994. [PubMed] [Google Scholar]
- Pinals R. S., Masi A. T., Larsen R. A. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981 Oct;24(10):1308–1315. doi: 10.1002/art.1780241012. [DOI] [PubMed] [Google Scholar]
- Quartier Pierre, Taupin Pierre, Bourdeaut Franck, Lemelle Irène, Pillet Pascal, Bost Michel, Sibilia Jean, Koné-Paut Isabelle, Gandon-Laloum Sylvie, LeBideau Marc. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003 Apr;48(4):1093–1101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]
- Reiff A., Takei S., Sadeghi S., Stout A., Shaham B., Bernstein B., Gallagher K., Stout T. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum. 2001 Jun;44(6):1411–1415. doi: 10.1002/1529-0131(200106)44:6<1411::AID-ART235>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ruperto N., Ravelli A., Falcini F., Lepore L., De Sanctis R., Zulian F., Buoncompagni A., Sardella M. L., Strano C., Alessio M. Performance of the preliminary definition of improvement in juvenile chronic arthritis patients treated with methotrexate. Italian Pediatric Rheumatology Study Group. Ann Rheum Dis. 1998 Jan;57(1):38–41. doi: 10.1136/ard.57.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeling H., Mathony K., John V., Keysser G., Burdach S., Horneff G. A combination of etanercept and methotrexate for the treatment of refractory juvenile idiopathic arthritis: a pilot study. Ann Rheum Dis. 2001 Apr;60(4):410–412. doi: 10.1136/ard.60.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief M. K., Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991 Aug 15;325(7):467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Sicotte N. L., Voskuhl R. R. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001 Nov 27;57(10):1885–1888. doi: 10.1212/wnl.57.10.1885. [DOI] [PubMed] [Google Scholar]
- Singh G., Athreya B. H., Fries J. F., Goldsmith D. P. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994 Dec;37(12):1761–1769. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- Takei S., Groh D., Bernstein B., Shaham B., Gallagher K., Reiff A. Safety and efficacy of high dose etanercept in treatment of juvenile rheumatoid arthritis. J Rheumatol. 2001 Jul;28(7):1677–1680. [PubMed] [Google Scholar]
- Woo P., Wedderburn L. R. Juvenile chronic arthritis. Lancet. 1998 Mar 28;351(9107):969–973. [PubMed] [Google Scholar]


