Abstract
Objectives: To describe the distribution of adipose tissue within and adjacent to entheses in order to assess its functional significance at attachment sites.
Methods: Entheses were removed from 29 different sites in the limbs of formalin fixed, elderly, dissecting room cadavers and the samples prepared for paraffin and/or methylmethacrylate histology. Entheses from four young volunteers with no history of significant musculoskeletal injury were examined by magnetic resonance imaging using T1 weighted sequences.
Results: Adipose tissue was present at several different sites at numerous entheses. Many tendons/ligaments lay on a bed of well vascularised, highly innervated, "insertional angle fat". Endotenon fat was striking between fascicles, where entheses flared out at their attachments. It was also characteristic of the epitenon, where it occurred in conjunction with lamellated and Pacinian corpuscles. Fat filled, meniscoid folds often protruded into joint cavities, immediately adjacent to attachment sites.
Conclusion: Adipose tissue is a common feature of normal entheses and should not be regarded as a sign of degeneration. It contributes to the increase in surface area of attachment sites, promotes movement between tendon/ligament and bone, and forms part of an enthesis organ that dissipates stress. The presence of numerous nerve endings in fat at attachment sites suggests that it has a mechanosensory role and this could account for the rich innervation of many entheses. Because damage to fat is known to lead to considerable joint pain, our findings may be important for understanding the site of pain in enthesopathies.
Full Text
The Full Text of this article is available as a PDF (535.0 KB).
Figure 1.
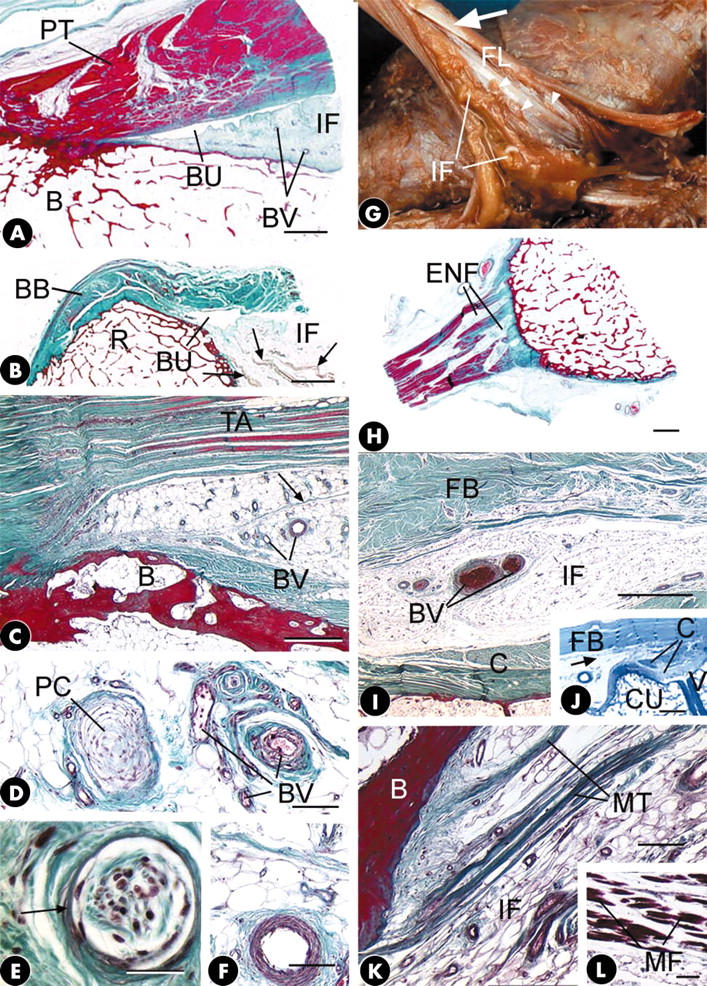
(A) The tibial enthesis of the patellar tendon (PT) showing the presence of "insertional angle fat" (IF) in the interval between the tendon and the bone (B) in association with the deep infrapatellar bursa (BU). BV, blood vessels. Masson's trichrome. Scale bar = 2 mm. (B) Insertional angle fat (IF) protruding into the bursa (BU) that lies deep to the tendon of biceps brachii (BB) at its attachment to the radial tuberosity (R). Note the occasional skeletal muscle fibres (arrows) running through the fat pad. Masson's trichrome. Scale bar = 4 mm. (C) Insertional angle fat filling the interval between the tendon of tibialis anterior (TA) and the bone (B) at its cuneiform attachment. Note the presence of numerous blood vessels (BV) and strands of fibrous tissue (arrow) in the fat. Masson's trichrome. Scale bar = 500 µm. (D) A small Pacinian corpuscle (PC) and numerous small blood vessels (BV) embedded in the insertional angle fat associated with the cuneiform attachment of the tendon of tibialis anterior. Masson's trichrome. Scale bar = 100 µm. (E) A lamellated corpuscle (arrow) surrounded by fibrous tissue in the insertional angle fat of biceps femoris at its fibular enthesis. Masson's trichrome. Scale bar = 30 µm. (F) A small muscular artery in the insertional angle fat at the cuneiform enthesis of tibialis anterior. Masson's trichrome. Scale bar = 100 µm. (G) Abundant insertional angle fat (IF) between the bone and the tendon of fibularis longus (FL) at its enthesis. The tendon has been cut and reflected so that its dorsal surface is visible. Note the spiralling of the tendon fascicles near its enthesis (arrow) and the increased surface area of the tendon near the bone. The insertional angle fat extends into the tendon as endotenon fat through gaps between the fascicles (arrowheads). (H) Conspicuous seams of endotenon fat (ENF) in the tendon of fibularis longus at its enthesis. Note how the tendon flares out as it approaches its bony interface. Masson's trichrome. Scale bar = 2 mm. (I) A transverse section of the tendon of fibularis brevis (FB) to show the layer of insertional angle fat (IF) that lies beneath the tendon and separates it from the capsule (C) of the 5th tarsometatarsal joint. Note the presence of blood vessels (BV) within this adipose tissue. Masson's trichrome. Scale bar = 1 mm. (J) A longitudinal section of the tendon of fibularis brevis (FB), near its metatarsal enthesis showing the presence of insertional angle fat (arrow). C, joint capsule; CU, cuboid; V, base of the 5th metatarsal. Toluidine blue. Scale bar = 2 mm. (K) Groups of collagen fibres that form microtendons (MT) running through the insertional angle fat (IF) and attaching to bone (B) at the enthesis of iliopsoas. Such microtendons are quite distinct from the macroscopic tendon (not shown) that attaches to the lesser trochanter. Masson's trichrome. Scale bar = 100 µm. (L) A short distance proximal to the microtendons featured in (K) are small numbers of skeletal muscle fibres (MF) that are attached to them. Masson's trichrome. Scale bar = 100 µm.
Figure 2.
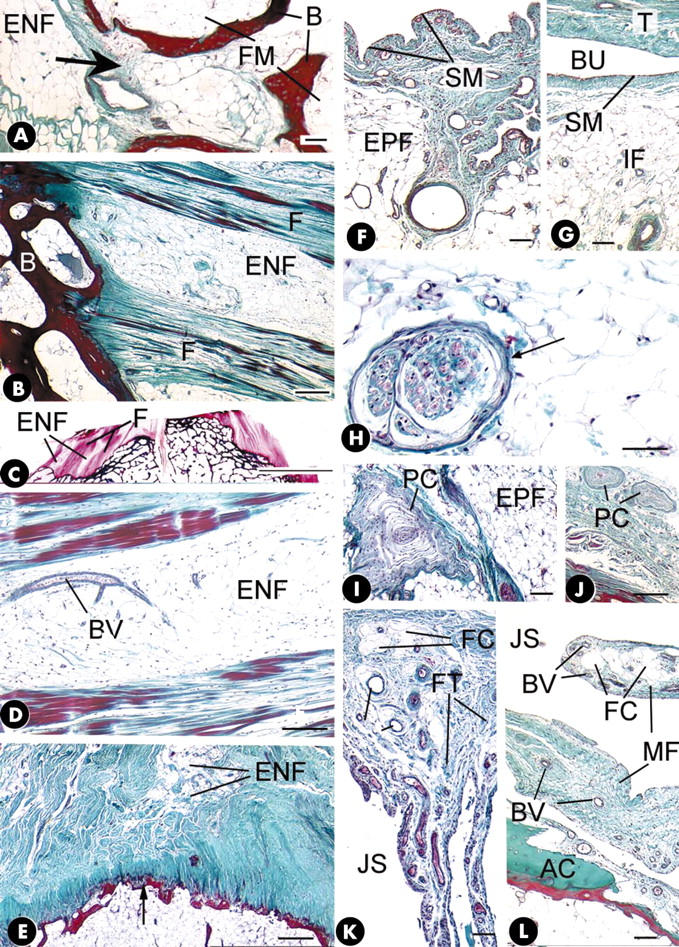
(A) Endotenon fat (ENF) at the attachment of fibularis longus that is continuous with fatty marrow (FM) through a small defect (arrow) in the subchondral bone (B) plate. Masson's trichrome. Scale bar = 100 µm. (B) Alternating seams of endotenon fat (ENF) and ligament fascicles (F) at the tibial enthesis of the anterior cruciate ligament. B, bone. Masson's trichrome. Scale bar = 300 µm. (C) A 100 µm thick methacrylate section of the tibial plateau showing seams of endotenon fat (ENF) between ligament fascicles (F) at the attachment of the anterior cruciate ligament. Azan. Scale bar = 1 cm. (D) A small blood vessel (BV) in the endotenon fat (ENF) at the tibial enthesis of the anterior cruciate ligament. Masson's trichrome. Scale bar = 200 µm. (E) The trochanteric enthesis of the tendon of gluteus medius. Note the presence of endotenon fat (ENF) opposite the point of maximum convexity of the bone surface (arrow); the tendon flares out to attach either side. Masson's trichrome. Scale bar = 500 µm. (F) Epitenon fat (EPF) on the surface of the anterior cruciate ligament lying beneath a synovial membrane (SM). Masson's trichrome. Scale bar = 100 µm. (G) Insertional angle fat (IF) covered in synovial membrane (SM) in the wall of the deep infrapatellar bursa (BU). T, tendon. Scale bar = 100 µm. (H) A lamellated corpuscle (arrow) in the epitenon fat on the surface of the posterior cruciate ligament at its tibial enthesis. Masson's trichrome. Scale bar = 50 µm. (I) A small Pacinian corpuscle (PC) embedded in epitenon fat (EPF) on the surface of the common extensor tendon at its lateral epicondylar enthesis. Masson's trichrome. Scale bar = 100 µm. (J) Two small Pacinian corpuscles (PC) in the fibroadipose tissue of the epitenon on the surface of the tendon of abductor pollicis brevis at its metacarpal enthesis. Masson's trichrome. Scale bar = 500 µm. (K) A meniscoid fold, covered with synovial membrane and containing a mixture of fat cells (FC), fibrous tissue (FT) and blood vessels, protruding into the joint space (JS) of the interphalangeal joint of the thumb at the insertion of the tendon of extensor pollicis longus. Masson's trichrome. Scale bar = 100 µm. (L) A meniscoid fold (MF) containing fat cells (FC) and blood vessels (BV) protruding into the tarsometatarsal joint space (JS) from the metatarsal enthesis of the tendon of tibialis anterior. AC, articular cartilage. Masson's trichrome. Scale bar = 100 µm.
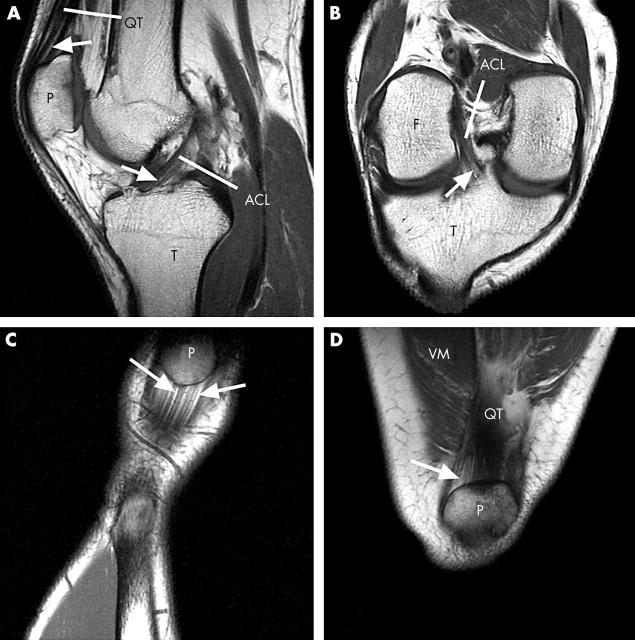
Figure 3.
(A) A sagittal MR image of the knee joint of a 29 year old man. Regions of endotenon fat (arrows) are conspicuous at the attachments of the quadriceps tendon (QT) to the patella (P) and the anterior cruciate ligament (ACL) to the tibia (T). (B) An oblique coronal MR image through the knee joint of the same subject showing endotenon fat (arrow) at the tibial enthesis of the anterior cruciate ligament (ACL). F, femur; T, tibia. (C) A coronal MR image of the proximal enthesis of the patellar tendon of a 39 year old woman showing seams of fat (arrows) in the endotenon extending from the bony interface. P, patella. (D) A coronal MR image of the attachment of the quadriceps tendon (QT) to the patella (P) in the same subject showing seams of fat (arrow) in the endotenon near the bony interface. VM, vastus medialis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin M., McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001 Nov;199(Pt 5):503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Qin S., Ralphs J. R. Fibrocartilage associated with human tendons and their pulleys. J Anat. 1995 Dec;187(Pt 3):625–633. [PMC free article] [PubMed] [Google Scholar]
- Biedert Roland M., Sanchis-Alfonso Vicente. Sources of anterior knee pain. Clin Sports Med. 2002 Jul;21(3):335-47, vii. doi: 10.1016/s0278-5919(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Bistevins R., Awad E. A. Structure and ultrastructure of mechanoreceptors at the human musculotendinous junction. Arch Phys Med Rehabil. 1981 Feb;62(2):74–83. [PubMed] [Google Scholar]
- Canoso J. J., Liu N., Traill M. R., Runge V. M. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988 Nov;47(11):910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Elena A., Kinsey Sally E., English Anne, Jones Richard A., Straszynski Liz, Meredith David M., Markham Alex F., Jack Andrew, Emery Paul, McGonagle Dennis. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002 Dec;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- Katonis P. G., Assimakopoulos A. P., Agapitos M. V., Exarchou E. I. Mechanoreceptors in the posterior cruciate ligament. Histologic study on cadaver knees. Acta Orthop Scand. 1991 Jun;62(3):276–278. doi: 10.3109/17453679108993609. [DOI] [PubMed] [Google Scholar]
- LaPrade R. F. The anatomy of the deep infrapatellar bursa of the knee. Am J Sports Med. 1998 Jan-Feb;26(1):129–132. doi: 10.1177/03635465980260010501. [DOI] [PubMed] [Google Scholar]
- Milz S., Putz R. Quantitative morphology of the subchondral plate of the tibial plateau. J Anat. 1994 Aug;185(Pt 1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Milz S., Rufai A., Buettner A., Putz R., Ralphs J. R., Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002 Feb;200(Pt 2):145–152. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl Bernhard, Kumai Tsukasa, Milz Stefan, Benjamin Michael. The structure and histopathology of the "enthesis organ" at the navicular insertion of the tendon of tibialis posterior. J Rheumatol. 2003 Mar;30(3):508–517. [PubMed] [Google Scholar]
- Narváez J. A., Narváez J., Ortega R., Aguilera C., Sánchez A., Andía E. Painful heel: MR imaging findings. Radiographics. 2000 Mar-Apr;20(2):333–352. doi: 10.1148/radiographics.20.2.g00mc09333. [DOI] [PubMed] [Google Scholar]
- Petrie S., Collins J., Solomonow M., Wink C., Chuinard R. Mechanoreceptors in the palmar wrist ligaments. J Bone Joint Surg Br. 1997 May;79(3):494–496. doi: 10.1302/0301-620x.79b3.7439. [DOI] [PubMed] [Google Scholar]
- Salingcarnboriboon R., Yoshitake H., Tsuji K., Obinata M., Amagasa T., Nifuji A., Noda M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003 Jul 15;287(2):289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Beattie J. H. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001 Aug;60(3):329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- Ushiyama T., Chano T., Inoue K., Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003 Feb;62(2):108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witoński D., Wagrowska-Danielewicz M. Distribution of substance-P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):177–183. doi: 10.1007/s001670050144. [DOI] [PubMed] [Google Scholar]
- Wojtys E. M., Beaman D. N., Glover R. A., Janda D. Innervation of the human knee joint by substance-P fibers. Arthroscopy. 1990;6(4):254–263. doi: 10.1016/0749-8063(90)90054-h. [DOI] [PubMed] [Google Scholar]