Abstract
Objectives: To investigate potential differences in phenotype and behaviour of immature (iDC) and mature dendritic cells (mDC) from patients with RA and healthy subjects.
Methods: iDC and mDC were derived from blood monocytes of patients with RA and healthy controls following standardised protocols. FACS was used to analyse expression of FcγRI, II, and III and molecules to characterise DC. Discrimination between FcγRIIa and FcγRIIb was achieved by RT-PCR. Immunohistochemistry was performed on synovial biopsy specimens of three patients with RA and three healthy controls. TNFα production by iDC and mDC upon FcγR dependent stimulation was compared between patients with RA and controls by ELISA.
Results: iDC from patients with active RA but not from patients with inactive RA or healthy controls markedly up regulated FcγRII. mDC from patients with active RA also lacked the physiological down regulation of FcγRII that occurs upon maturation in both control groups. RT-PCR analysis confirmed the increased expression of FcγRII in RA—especially marked for FcγRIIb. FcγR dependent stimulation of DC using antigen-IgG immune complexes (IC) significantly increased TNFα production by DC from healthy subjects, but significantly decreased TNFα by DC from patients with RA. Overlapping expression patterns between FcγRII and DC-LAMP in the synovial tissue of patients with RA imply that in vivo, also, mature DC express increased levels of FcγRIIb.
Conclusion: The presence and altered characteristics of DC during active RA suggest that DC help to modulate autoimmunity in RA. Further studies should elucidate the role of local factors in altering the function of DC in RA and in increasing expression of FcγRII.
Full Text
The Full Text of this article is available as a PDF (329.6 KB).
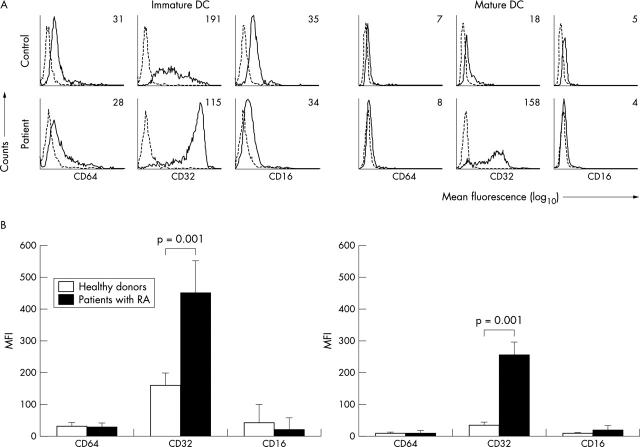
Figure 1.
FcγRI, II, and III expression on iDC and mDC from patients with RA and healthy subjects. (A) FACS analysis of the indicated markers (solid line) or isotype controls (dotted line) of DC within the life gate. Numbers within the histograms represent the mean fluorescence of the marker corrected for isotype values. Each graph displays data from one representative donor. (B) Averaged mean expression of the indicated markers from the whole group of healthy donors (n = 32) and patients with RA (n = 31) of both iDC and mDC.
Figure 2.
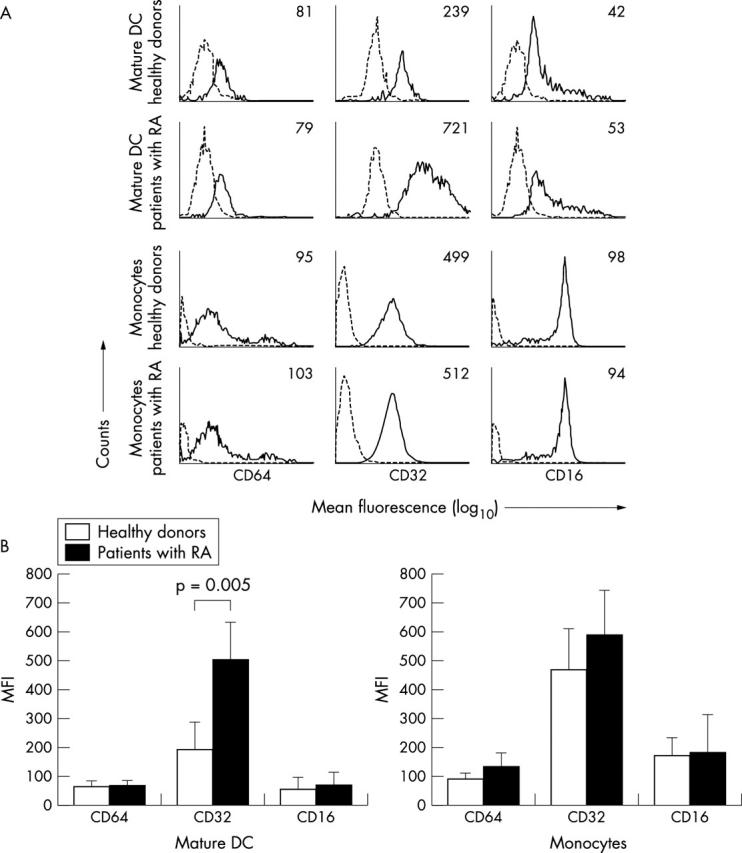
Expression of FcγRI, II, and III on mDC and monocytes from patients with RA and healthy subjects using double staining techniques. (A) MFI of the FcγR subtypes (solid line) and isotype control (dotted line) on mDC (CD83, FcγR double positive cells) and monocytes (CD14, FcγR double positive cells) within the life gate by using double staining FACS analysis. The mean fluorescence is indicated within the histogram. (B) Averaged mean expression of the indicated markers on mDC and monocytes from the whole group of healthy donors (n = 9, n = 10) and patients with RA (n = 13, n = 10), respectively. Of note, only CD83, FcγR and CD14, FcγR double positive cells are shown.
Figure 3.
FcγRI, II, and III expression by iDC and influence of RA disease activity. (A) FACS analysis of FcγR subtypes (solid line) and isotype control (dotted line) on iDC from a healthy donor, a patient with active RA, and a patient with RA in remission. The numbers within the histograms indicate the mean fluorescence. Each histogram displays one representative person. (B) Averaged mean expression of CD64, CD32, and CD16 (FcγRI, II, and III, respectively) on iDC from healthy donors (n = 10), patients with RA in remission (n = 6), and patients with RA with active disease (n = 8).
Figure 4.
mRNA expression of FcγRIIa and FcγRIIb by iDC and mDC from patients with RA and healthy controls. The bars represent the median level of FcγRII mRNA analysed with PCR techniques, corrected for the expression of the housekeeping gene GAPDH. Eight patients with active RA, six patients with RA in remission (n = 6), and 10 healthy donors (n = 10) were studied.
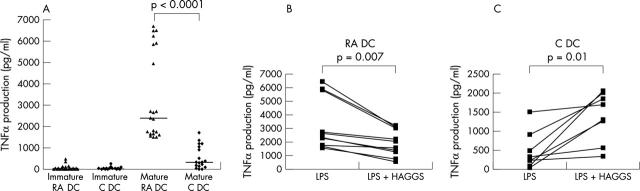
Figure 5.
(A) TNFα production by iDC and mDC. TNFα production (pg/ml) by iDC (n = 21) and mDC (n = 21) from patients with RA (RA DC) and DC from healthy controls (n = 18) (C DC). Full maturation was achieved by adding LPS on day 6. (B) TNFα production by mDC after stimulation with anti-IgG complexes (HAGGs). TNFα production of mDC with and without HAGGs stimulation from patients with RA (n = 10, B) and from healthy subjects (n = 8, C). Full maturation was achieved by adding LPS on day 6.
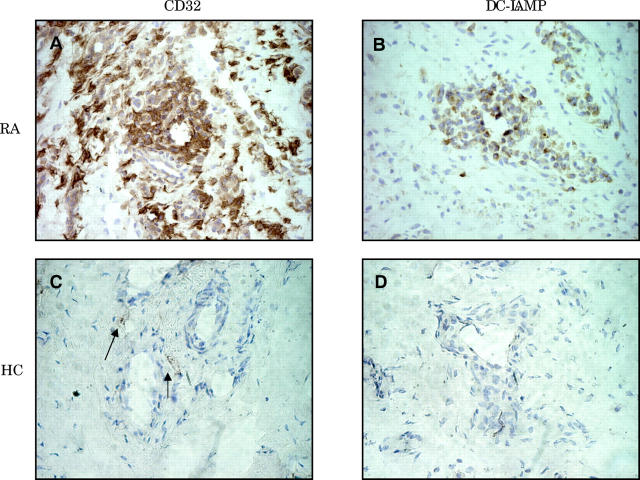
Figure 6.
Co-expression of FcγRII and CD83 in synovial tissue. (A) and (B) show immunostaining of RA synovial tissue with CD32 and DC-LAMP, respectively. Immunostaining with the same set of markers is shown for synovial tissue of a healthy control (HC), respectively.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams V. M., Cambridge G., Lydyard P. M., Edwards J. C. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000 Mar;43(3):608–616. doi: 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Adema G. J., Hartgers F., Verstraten R., de Vries E., Marland G., Menon S., Foster J., Xu Y., Nooyen P., McClanahan T. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997 Jun 12;387(6634):713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- Amigorena Sebastian. Fc gamma receptors and cross-presentation in dendritic cells. J Exp Med. 2002 Jan 7;195(1):F1–F3. doi: 10.1084/jem.20011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bell D., Young J. W., Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Blom Arjen B., Radstake Timothy R. D. J., Holthuysen Astrid E. M., Slöetjes Annet W., Pesman Gerard J., Sweep Fred G. J., van de Loo Fons A. J., Joosten L. A. B., Barrera Pilar, van Lent Peter L. E. M. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003 Apr;48(4):1002–1014. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- Cassel D. L., Keller M. A., Surrey S., Schwartz E., Schreiber A. D., Rappaport E. F., McKenzie S. E. Differential expression of Fc gamma RIIA, Fc gamma RIIB and Fc gamma RIIC in hematopoietic cells: analysis of transcripts. Mol Immunol. 1993 Apr;30(5):451–460. doi: 10.1016/0161-5890(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Dijstelbloem H. M., van de Winkel J. G., Kallenberg C. G. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 2001 Sep;22(9):510–516. doi: 10.1016/s1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- Fanger N. A., Wardwell K., Shen L., Tedder T. F., Guyre P. M. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J Immunol. 1996 Jul 15;157(2):541–548. [PubMed] [Google Scholar]
- Feldmann M., Charles P., Taylor P., Maini R. N. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol. 1998;20(1-2):211–228. doi: 10.1007/BF00832008. [DOI] [PubMed] [Google Scholar]
- Figdor Carl G., van Kooyk Yvette, Adema Gosse J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002 Feb;2(2):77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B., Torensma R., van Vliet S. J., van Duijnhoven G. C., Adema G. J., van Kooyk Y., Figdor C. G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000 Mar 3;100(5):575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Bonder C. S., Balogh J., Dickensheets H. L., Donnelly R. P., Finlay-Jones J. J. Differential responses of human monocytes and macrophages to IL-4 and IL-13. J Leukoc Biol. 1999 Oct;66(4):575–578. [PubMed] [Google Scholar]
- Isomäki P., Luukkainen R., Toivanen P., Punnonen J. The presence of interleukin-13 in rheumatoid synovium and its antiinflammatory effects on synovial fluid macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1996 Oct;39(10):1693–1702. doi: 10.1002/art.1780391012. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Ollier W., Awad J., Young A., Silman A., Roitt I. M., Corbett M., Hay F., Cosh J. A., Maini R. N. HLA and rheumatoid arthritis: a combined analysis of 440 British patients. Ann Rheum Dis. 1986 Aug;45(8):627–636. doi: 10.1136/ard.45.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Hiroto, Lian Zhe-Xiong, Van de Water Judy, He Xiao-Song, Matsumura Shuji, Kaplan Marshall, Luketic Velimir, Coppel Ross L., Ansari Aftab A., Gershwin M. Eric. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002 Jan 7;195(1):113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau S., Martinsson P., Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000 May 1;191(9):1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001 Aug 10;106(3):263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Davis D., Macfarlane J. D., Antoni C., Leeb B., Elliott M. J., Woody J. N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Malbec O., Fridman W. H., Daëron M. Negative regulation of hematopoietic cell activation and proliferation by Fc gamma RIIB. Curr Top Microbiol Immunol. 1999;244:13–27. [PubMed] [Google Scholar]
- Mellman I., Steinman R. M. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001 Aug 10;106(3):255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Yuasa T., Ujike A., Ono M., Nukiwa T., Ravetch J. V., Takai T. Fcgamma receptor IIB-deficient mice develop Goodpasture's syndrome upon immunization with type IV collagen: a novel murine model for autoimmune glomerular basement membrane disease. J Exp Med. 2000 Mar 6;191(5):899–906. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page Guillaume, Lebecque Serge, Miossec Pierre. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002 May 15;168(10):5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Pettit A. R., MacDonald K. P., O'Sullivan B., Thomas R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum. 2000 Apr;43(4):791–800. doi: 10.1002/1529-0131(200004)43:4<791::AID-ANR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Pricop L., Redecha P., Teillaud J. L., Frey J., Fridman W. H., Sautès-Fridman C., Salmon J. E. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001 Jan 1;166(1):531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- Qin D., Wu J., Vora K. A., Ravetch J. V., Szakal A. K., Manser T., Tew J. G. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000 Jun 15;164(12):6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Salmon J. E., Pricop L. Human receptors for immunoglobulin G: key elements in the pathogenesis of rheumatic disease. Arthritis Rheum. 2001 Apr;44(4):739–750. doi: 10.1002/1529-0131(200104)44:4<739::AID-ANR129>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman Ralph Marvin, Nussenzweig Michel C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002 Jan 2;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. L., Daniel P. B., O'Donnell J. L., Hart D. N. Dendritic cells in synovial fluid of chronic inflammatory arthritis lack CD80 surface expression. Clin Exp Immunol. 1995 Apr;100(1):81–89. doi: 10.1111/j.1365-2249.1995.tb03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B., Corper A., Bonagura V., Taussig M. The structure and origin of rheumatoid factors. Immunol Today. 2000 Apr;21(4):177–183. doi: 10.1016/s0167-5699(00)01589-9. [DOI] [PubMed] [Google Scholar]
- Sweep C. G., Geurts-Moespot J., Grebenschikov N., de Witte J. H., Heuvel J. J., Schmitt M., Duffy M. J., Jänicke F., Kramer M. D., Foekens J. A. External quality assessment of trans-European multicentre antigen determinations (enzyme-linked immunosorbent assay) of urokinase-type plasminogen activator (uPA) and its type 1 inhibitor (PAI-1) in human breast cancer tissue extracts. Br J Cancer. 1998 Dec;78(11):1434–1441. doi: 10.1038/bjc.1998.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994 Mar 1;152(5):2613–2623. [PubMed] [Google Scholar]
- Thomas R., Lipsky P. E. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996 Dec;17(12):559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T., Kubo S., Yoshino T., Ujike A., Matsumura K., Ono M., Ravetch J. V., Takai T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999 Jan 4;189(1):187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995 Apr 15;154(8):3821–3835. [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B., Vincent J., Vandenabeele S., Vanbervliet B., Pin J. J., Aït-Yahia S., Patel S., Mattei M. G., Banchereau J., Zurawski S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998 Sep;9(3):325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., van Vuuren A. J., Blom A. B., Holthuysen A. E., van de Putte L. B., van de Winkel J. G., van den Berg W. B. Role of Fc receptor gamma chain in inflammation and cartilage damage during experimental antigen-induced arthritis. Arthritis Rheum. 2000 Apr;43(4):740–752. doi: 10.1002/1529-0131(200004)43:4<740::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]