Abstract
Background: Leukotriene B4 (LTB4) has a key role in the pathophysiology of rheumatoid arthritis (RA).
Objective: To investigate the inhibition of ex vivo LTB4-induced Mac-1 (CD11b/CD18) expression in leucocytes of patients with RA by the new oral LTB4 receptor antagonist BIIL 284.
Methods: The pharmacokinetics and inhibition of LTB4-induced Mac-1 expression of BIIL 284 were characterised in 26 adult patients with RA who were treated with BIIL 284 25 mg, 150 mg, or placebo given once a day for 14 days according to a double blind, randomised, parallel group design.
Results: Tmax of BIIL 315 in plasma (main metabolite and active principle of BIIL 284 in plasma) was achieved about four hours after drug administration, and Cmax,ss and AUC0–6h,ss increased in proportion to the dosage. 100% inhibition of LTB4-induced MAC-1 expression was reached after two hours (150 mg) or four hours (25 mg), showing a statistically significant difference in comparison with placebo (p<0.005). A longlasting dynamic effect was seen consistently even when plasma concentrations declined to very low values 24 hours after administration. Secondary clinical efficacy end points remained unchanged probably owing to the short duration of treatment. Adverse events (AEs) were reported in 12 patients during the study. No serious AEs or laboratory AEs were seen.
Conclusions: Both the 25 mg and 150 mg doses of BIIL 284 safely and effectively inhibit Mac-1 expression on neutrophils; thus longer treatment with BIIL 284 may result in clinical benefit for patients with RA.
Full Text
The Full Text of this article is available as a PDF (293.1 KB).
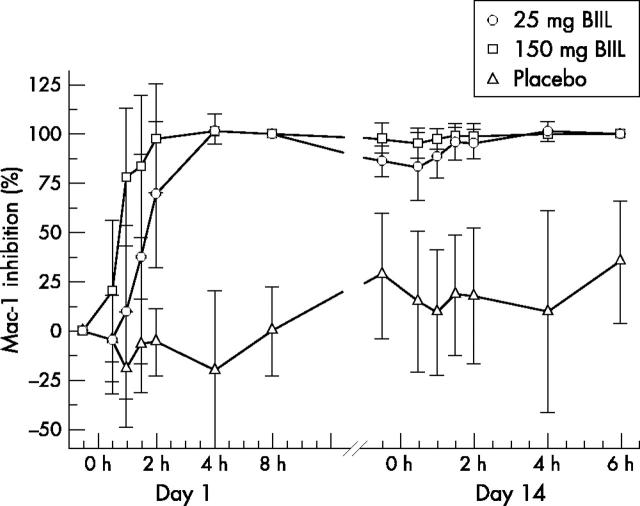
Figure 1 .
Inhibition of ex vivo LTB4 IME (%) over time. Mean and SD of inhibition are plotted for the different treatment groups (n = 7 for 25 mg BIIL 284, n = 8 for 150 mg BIIL 284, n = 9 for placebo).
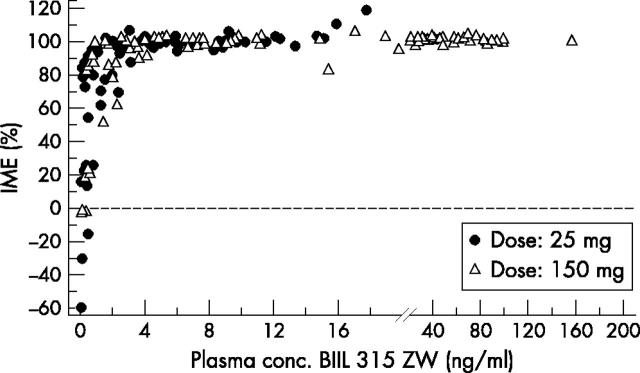
Figure 2 .
Relationship between BIIL 284 315 ZW plasma concentrations and inhibition of ex vivo LTB4 induced Mac-1 expression (IME) on neutrophils. Summarised data after once daily oral administration of 25 mg (n = 7) and 150 mg (n = 8) BIIL 284 for all time points on day 1 and day 14.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Birke F. W., Meade C. J., Anderskewitz R., Speck G. A., Jennewein H. M. In vitro and in vivo pharmacological characterization of BIIL 284, a novel and potent leukotriene B(4) receptor antagonist. J Pharmacol Exp Ther. 2001 Apr;297(1):458–466. [PubMed] [Google Scholar]
- Chen X. S., Sheller J. R., Johnson E. N., Funk C. D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994 Nov 10;372(6502):179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. M., Rae S. A., Smith M. J. Leukotriene B4, a mediator of inflammation present in synovial fluid in rheumatoid arthritis. Ann Rheum Dis. 1983 Dec;42(6):677–679. doi: 10.1136/ard.42.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmgreen J., Nielsen O. H., Ahnfelt-Rønne I. Enhanced capacity for release of leucotriene B4 by neutrophils in rheumatoid arthritis. Ann Rheum Dis. 1987 Jul;46(7):501–505. doi: 10.1136/ard.46.7.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D. T., Anderson J. J., Boers M., Bombardier C., Furst D., Goldsmith C., Katz L. M., Lightfoot R., Jr, Paulus H., Strand V. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995 Jun;38(6):727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. Leukotriene B4 in inflammation. Crit Rev Immunol. 1990;10(1):1–12. [PubMed] [Google Scholar]
- Griffiths R. J., Pettipher E. R., Koch K., Farrell C. A., Breslow R., Conklyn M. J., Smith M. A., Hackman B. C., Wimberly D. J., Milici A. J. Leukotriene B4 plays a critical role in the progression of collagen-induced arthritis. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):517–521. doi: 10.1073/pnas.92.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. J., Smith M. A., Roach M. L., Stock J. L., Stam E. J., Milici A. J., Scampoli D. N., Eskra J. D., Byrum R. S., Koller B. H. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J Exp Med. 1997 Mar 17;185(6):1123–1129. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürsel T., Firat S., Ercan Z. S. Increased serum leukotriene B4 level in the active stage of rheumatoid arthritis in children. Prostaglandins Leukot Essent Fatty Acids. 1997 Mar;56(3):205–207. doi: 10.1016/s0952-3278(97)90535-4. [DOI] [PubMed] [Google Scholar]
- Hubbard R. C., Fells G., Gadek J., Pacholok S., Humes J., Crystal R. G. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991 Sep;88(3):891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Shapleigh C., Goetzl E. J. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J Clin Invest. 1980 Nov;66(5):1166–1170. doi: 10.1172/JCI109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshihara Y., Isono T., Oda H., Karube S., Hayashi Y. Measurement of sulfidopeptide leukotrienes and their metabolism in human synovial fluid of patients with rheumatoid arthritis. Prostaglandins Leukot Essent Fatty Acids. 1988 Jun;32(3):113–119. [PubMed] [Google Scholar]
- Lee E., Lindo T., Jackson N., Meng-Choong L., Reynolds P., Hill A., Haswell M., Jackson S., Kilfeather S. Reversal of human neutrophil survival by leukotriene B(4) receptor blockade and 5-lipoxygenase and 5-lipoxygenase activating protein inhibitors. Am J Respir Crit Care Med. 1999 Dec;160(6):2079–2085. doi: 10.1164/ajrccm.160.6.9903136. [DOI] [PubMed] [Google Scholar]
- Leppert D., Hauser S. L., Kishiyama J. L., An S., Zeng L., Goetzl E. J. Stimulation of matrix metalloproteinase-dependent migration of T cells by eicosanoids. FASEB J. 1995 Nov;9(14):1473–1481. doi: 10.1096/fasebj.9.14.7589989. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Moilanen E. Prostanoids and leukotrienes in rheumatoid synovitis. Pharmacol Toxicol. 1994;75 (Suppl 2):4–8. doi: 10.1111/j.1600-0773.1994.tb01989.x. [DOI] [PubMed] [Google Scholar]
- O'Dell J. R. Anticytokine therapy--a new era in the treatment of rheumatoid arthritis? N Engl J Med. 1999 Jan 28;340(4):310–312. doi: 10.1056/NEJM199901283400411. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Bouvrette L., Gingras D., Girard M. Identification of interferon-gamma as the lymphokine that mediates leukotriene B4-induced immunoregulation. J Immunol. 1987 Jul 15;139(2):513–517. [PubMed] [Google Scholar]
- Sperling R. I., Benincaso A. I., Anderson R. J., Coblyn J. S., Austen K. F., Weinblatt M. E. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum. 1992 Apr;35(4):376–384. doi: 10.1002/art.1780350403. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kremer J. M., Coblyn J. S., Helfgott S., Maier A. L., Petrillo G., Henson B., Rubin P., Sperling R. Zileuton, a 5-lipoxygenase inhibitor in rheumatoid arthritis. J Rheumatol. 1992 Oct;19(10):1537–1541. [PubMed] [Google Scholar]
- Wijnands M. J., van Riel P. L. Management of adverse effects of disease-modifying antirheumatic drugs. Drug Saf. 1995 Oct;13(4):219–227. doi: 10.2165/00002018-199513040-00002. [DOI] [PubMed] [Google Scholar]
- Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997 Jun 5;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- van Gestel A. M., Haagsma C. J., van Riel P. L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998 Oct;41(10):1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- van Pelt J. P., de Jong E. M., van Erp P. E., Mitchell M. I., Marder P., Spaethe S. M., van Hooijdonk C. A., Kuijpers A. L., van de Kerkhof P. C. The regulation of CD11b integrin levels on human blood leukocytes and leukotriene B4-stimulated skin by a specific leukotriene B4 receptor antagonist (LY293111). Biochem Pharmacol. 1997 Apr 4;53(7):1005–1012. doi: 10.1016/s0006-2952(96)00884-2. [DOI] [PubMed] [Google Scholar]