Abstract
Background: Randomised controlled trials have shown that treatment with anti-tumour necrosis factor (anti-TNF) agents is effective in refractory rheumatoid arthritis (RA).
Objective: To determine the effectiveness of anti-TNF in a general unselected group of patients with refractory RA.
Methods: 68 patients with active RA despite treatment with disease modifying antirheumatic drugs were studied during 12 infliximab infusions. Infliximab (3 mg/kg/infusion) was given every 8 or 6 weeks. Clinical efficacy was assessed by the Disease Activity Score (DAS) index (44 joints). Dose adjustments were based on residual disease activity (DAS score >2.4). The primary end points were the percentage of patients achieving good or moderate response by the EULAR response criteria and the proportion of patients requiring dose adjustment.
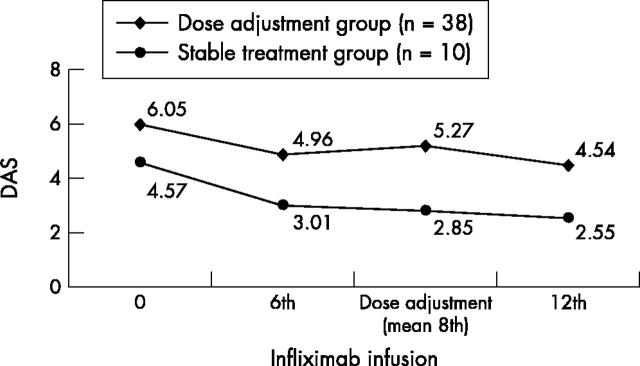
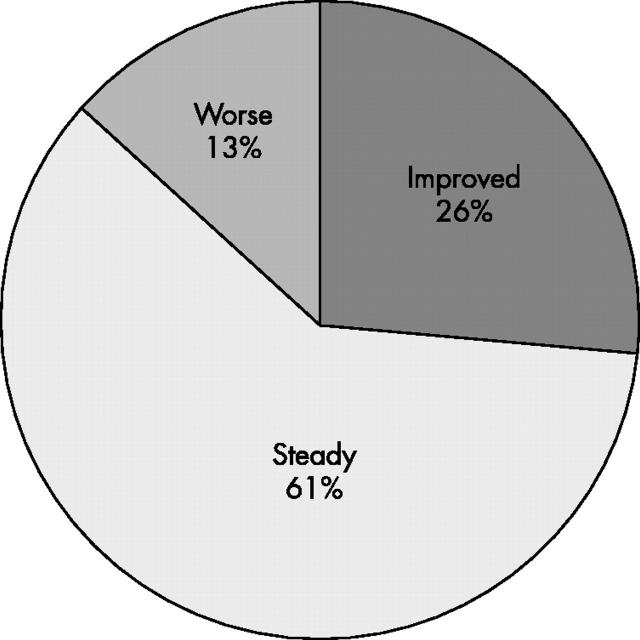
Results: 20 (29%) patients discontinued treatment owing to side effects, early inefficacy, or other considerations. Among the patients who continued treatment, 27 (56%) and 32 (67%) were responders on the 6th and 12th infliximab infusion, respectively. In the same patients, disease activity gradually improved without modifications in the initial dosing in 10 (21%), whereas in 38 (79%) the dose of infliximab and/or methotrexate was increased. Intensification of treatment led to a significant decrease in the mean DAS score in this group (from 5.27 just before dose modification to 4.54 before the 12th infusion, p<0.002). The EULAR response category improved in only 10/38 (26%), however.
Conclusions: In this initial observational study of patients with RA treated with recommended doses of infliximab, adjustments in treatment were common but not always sufficient to maintain adequate disease control. Longitudinal controlled trials are needed to define the optimal dose escalation in patients with suboptimal response.
Full Text
The Full Text of this article is available as a PDF (269.0 KB).
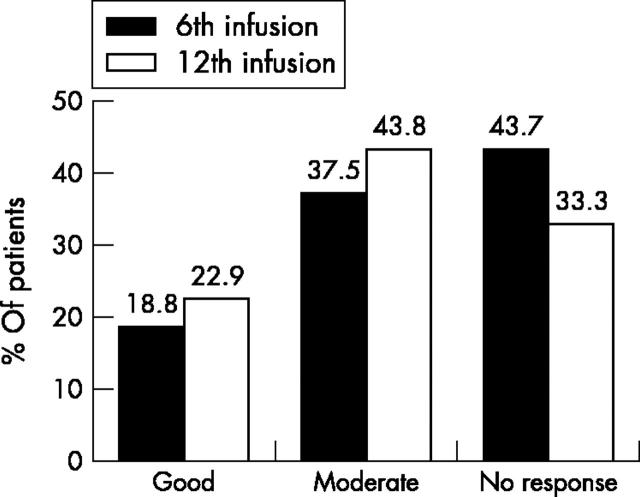
Figure 1 .
Response rates at the 6th and 12th infusions of infliximab according to EULAR response criteria (% of patients).
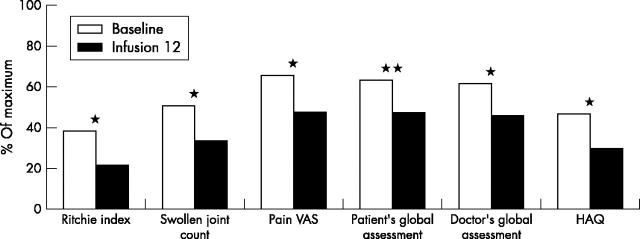
Figure 2 .
Improvement in clinical parameters (n = 48). *p<0.001; **p<0.002.
Figure 3 .
Mean DAS over time for each group of patients: the first with stable treatment (n = 10) and the second with treatment modification (n = 38).
Figure 4 .
Changes in EULAR response categories after treatment adjustments (n = 38).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baert Filip, Noman Maja, Vermeire Severine, Van Assche Gert, D' Haens Geert, Carbonez An, Rutgeerts Paul. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003 Feb 13;348(7):601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- Den Broeder A. A., Creemers M. C. W., van Gestel A. M., van Riel P. L. C. M. Dose titration using the Disease Activity Score (DAS28) in rheumatoid arthritis patients treated with anti-TNF-alpha. Rheumatology (Oxford) 2002 Jun;41(6):638–642. doi: 10.1093/rheumatology/41.6.638. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Kalden J. R., Antoni C., Smolen J. S., Leeb B., Breedveld F. C., Macfarlane J. D., Bijl H. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Fransen J., Stucki G., van Riel P. The merits of monitoring: should we follow all our rheumatoid arthritis patients in daily practice? Rheumatology (Oxford) 2002 Jun;41(6):601–604. doi: 10.1093/rheumatology/41.6.601. [DOI] [PubMed] [Google Scholar]
- Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Antoni C. E., Bijlsma J. W. J., Burmester G. R., Cronstein B., Keystone E. C., Kavanaugh A. Updated consensus statement on biological agents for the treatment of rheumatoid arthritis and other rheumatic diseases (May 2002). Ann Rheum Dis. 2002 Nov;61 (Suppl 2):ii2–ii7. doi: 10.1136/ard.61.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel Sherine E., Crowson Cynthia S., Kremers Hilal Maradit, Doran Michele F., Turesson Carl, O'Fallon W. Michael, Matteson Eric L. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003 Jan;48(1):54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- Geborek P., Crnkic M., Petersson I. F., Saxne T., South Swedish Arthritis Treatment Group Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis. 2002 Sep;61(9):793–798. doi: 10.1136/ard.61.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. J., Wolfe F. Are the results of controlled clinical trials and observational studies of second line therapy in rheumatoid arthritis valid and generalizable as measures of rheumatoid arthritis outcome: analysis of 122 studies. J Rheumatol. 1991 Jul;18(7):1008–1014. [PubMed] [Google Scholar]
- Hochberg M. C., Chang R. W., Dwosh I., Lindsey S., Pincus T., Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992 May;35(5):498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., van der Heijde D. M., St Clair E. W., Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Weisman M., Emery P., Feldmann M. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000 Nov 30;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Davis D., Macfarlane J. D., Antoni C., Leeb B., Elliott M. J., Woody J. N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Maini R., St Clair E. W., Breedveld F., Furst D., Kalden J., Weisman M., Smolen J., Emery P., Harriman G., Feldmann M. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999 Dec 4;354(9194):1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Pincus T. Limitations of randomized controlled clinical trials to depict accurately long-term outcomes in rheumatoid arthritis. Z Rheumatol. 1998 Feb;57(1):46–49. doi: 10.1007/s003930050059. [DOI] [PubMed] [Google Scholar]
- Smolen J. S., Breedveld F. C., Schiff M. H., Kalden J. R., Emery P., Eberl G., van Riel P. L., Tugwell P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003 Feb;42(2):244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kremer J. M., Bankhurst A. D., Bulpitt K. J., Fleischmann R. M., Fox R. I., Jackson C. G., Lange M., Burge D. J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999 Jan 28;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- Weinblatt Michael E., Keystone Edward C., Furst Daniel E., Moreland Larry W., Weisman Michael H., Birbara Charles A., Teoh Leah A., Fischkoff Steven A., Chartash Elliot K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003 Jan;48(1):35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- Welsing P. M., van Gestel A. M., Swinkels H. L., Kiemeney L. A., van Riel P. L. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001 Sep;44(9):2009–2017. doi: 10.1002/1529-0131(200109)44:9<2009::AID-ART349>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- van Gestel A. M., Prevoo M. L., van 't Hof M. A., van Rijswijk M. H., van de Putte L. B., van Riel P. L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996 Jan;39(1):34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- van Riel P. L., van Gestel A. M. Clinical outcome measures in rheumatoid arthritis. Ann Rheum Dis. 2000 Nov;59 (Suppl 1):i28–i31. doi: 10.1136/ard.59.suppl_1.i28. [DOI] [PMC free article] [PubMed] [Google Scholar]