Abstract
Objective: To determine whether overexpression of cell membrane associated drug efflux pumps belonging to the family of ATP binding cassette (ABC) proteins contributes to a diminished efficacy of sulfasalazine (SSZ) after prolonged cellular exposure to this disease modifying antirheumatic drug (DMARD).
Methods: A model system of human T cells (CEM) was used to expose cells in vitro to increasing concentrations of SSZ for a period of six months. Cells were then characterised for the expression of drug efflux pumps: P-glycoprotein (Pgp, ABCB1), multidrug resistance protein 1 (MRP1, ABCC1), and breast cancer resistance protein (BCRP, ABCG2).
Results: Prolonged exposure of CEM cells to SSZ provoked resistance to SSZ as manifested by a 6.4-fold diminished antiproliferative effect of SSZ compared with parental CEM cells. CEM cells resistant to SSZ (CEM/SSZ) showed a marked induction of ABCG2/BCRP, Pgp expression was not detectable, while MRP1 expression was even down regulated. A functional role of ABCG2 in SSZ resistance was demonstrated by 60% reversal of SSZ resistance by the ABCG2 blocker Ko143. Release of the proinflammatory cytokine tumour necrosis factor α (TNFα) was threefold higher in CEM/SSZ cells than in CEM cells. Moreover, twofold higher concentrations of SSZ were required to inhibit TNFα release from CEM/SSZ cells compared with CEM cells.
Conclusion: Collectively, ABCG2 induction, augmented TNFα release, and less efficient inhibition of TNFα production by SSZ may contribute to diminished efficacy after prolonged exposure to SSZ. These results warrant further clinical studies to verify whether drug efflux pumps, originally identified for their roles in cytostatic drug resistance, can also be induced by SSZ or other DMARDs.
Full Text
The Full Text of this article is available as a PDF (298.8 KB).
Figure 1 .
(A) Onset of SSZ resistance in human CEM (T) cells. SSZ resistance was provoked by culturing CEM (T) cells in stepwise increasing concentrations SSZ over time. (B) SSZ sensitivity for CEM (T) cells, CEM/SSZ1.5, and CEM/SSZ2.5 cells. Antiproliferative effects were assessed after 72 hours' exposure to SSZ. Results are the mean of 3–5 separate experiments (SD <20%).
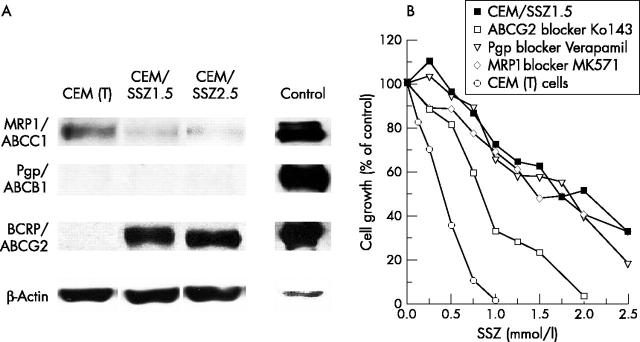
Figure 2 .
(A) Expression of MDR transporters Pgp, MRP1, and ABCG2/BCRP in CEM (T) and CEM/SSZ1.5 cells. Western blots were performed using total cell lysates of CEM (T) and CEM/SSZ1.5 cells. Cell lysates of CEM/VBL100,64 2008/MRP1,32 and MCF7/MR40 served as positive control for Pgp, MRP1, and ABCG2, respectively. For CEM (T) and CEM/SSZ1.5 cells, 50 µg of cell lysate was applied on the sodium dodecyl sulphate-polyacrylamide gel, and for the controls 10 µg protein. Pgp was detected by the monoclonal antibody JSB1, MRP1 was detected by the monoclonal antibody MRPr1, and ABCG2 was detected by the monoclonal antibody BXP21.32,33 (B) Reversal of SSZ resistance in CEM/SSZ1.5 cells by ABCG2 blocker Ko143, but not by Pgp blocker verapamil or MRP1 blocker MK571. CEM/SSZ1.5 cells were incubated in medium containing non-toxic concentrations of the ABCG2 blocker Ko143 (0.5 µM), MRP1 blocker MK571 (20 µM), or Pgp blocker verapamil (10 µM) and evaluated for SSZ sensitivity compared with parental CEM (T) cells as described in fig 1B. Results are presented as the mean of three experiments (SD <20%).
Figure 3 .
(A) TNFα production by CEM (T), CEM/SSZ1.5, and CEM/SSZ2.5 cells (3x105/ml) after 24 hours' stimulation with PMA/ionomycin in the absence or presence of a concentration range of SSZ. TNFα levels were determined by ELISA. Results are mean (SD) of 5–7 separate experiments. (B) NFkB p65 expression in nuclear extracts of CEM (T) cells and CEM/SSZ1.5 cells after 24 hours' stimulation with PMA/ionomycin in the absence or presence of SSZ (1.0 mM).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen John D., Schinkel Alfred H. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2). Mol Cancer Ther. 2002 Apr;1(6):427–434. [PubMed] [Google Scholar]
- Allen John D., van Loevezijn Arnold, Lakhai Jeany M., van der Valk Martin, van Tellingen Olaf, Reid Glen, Schellens Jan H. M., Koomen Gerrit-Jan, Schinkel Alfred H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002 Apr;1(6):417–425. [PubMed] [Google Scholar]
- Ambudkar S. V., Dey S., Hrycyna C. A., Ramachandra M., Pastan I., Gottesman M. M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Johnson M. A. Inhibition by sulfasalazine of LTC synthetase and of rat liver glutathione S-transferases. Biochem Pharmacol. 1985 Aug 1;34(15):2695–2704. doi: 10.1016/0006-2952(85)90570-2. [DOI] [PubMed] [Google Scholar]
- Bailey-Dell K. J., Hassel B., Doyle L. A., Ross D. D. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta. 2001 Sep 21;1520(3):234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001 Feb;107(3):241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera P., Haagsma C. J., Boerbooms A. M., Van Riel P. L., Borm G. F., Van de Putte L. B., Van der Meer J. W. Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol. 1995 Aug;34(8):747–755. doi: 10.1093/rheumatology/34.8.747. [DOI] [PubMed] [Google Scholar]
- Baz A., Henry L., Caravano R., Scherrer K., Bureau J. P. Changes in the subunit distribution of prosomes (MCP-proteasomes) during the differentiation of human leukemic cells. Int J Cancer. 1997 Jul 29;72(3):467–476. doi: 10.1002/(sici)1097-0215(19970729)72:3<467::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Cirtain M. C., Look A. T., Ashmun R. A. Reversal of Vinca alkaloid resistance but not multiple drug resistance in human leukemic cells by verapamil. Cancer Res. 1986 Feb;46(2):778–784. [PubMed] [Google Scholar]
- Boers M., Verhoeven A. C., Markusse H. M., van de Laar M. A., Westhovens R., van Denderen J. C., van Zeben D., Dijkmans B. A., Peeters A. J., Jacobs P. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997 Aug 2;350(9074):309–318. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- Boland M. P., Fitzgerald K. A., O'Neill L. A. Topoisomerase II is required for mitoxantrone to signal nuclear factor kappa B activation in HL60 cells. J Biol Chem. 2000 Aug 18;275(33):25231–25238. doi: 10.1074/jbc.275.33.25231. [DOI] [PubMed] [Google Scholar]
- Boland M. P., Foster S. J., O'Neill L. A. Daunorubicin activates NFkappaB and induces kappaB-dependent gene expression in HL-60 promyelocytic and Jurkat T lymphoma cells. J Biol Chem. 1997 May 16;272(20):12952–12960. doi: 10.1074/jbc.272.20.12952. [DOI] [PubMed] [Google Scholar]
- Borst P., Elferink R. Oude. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2001 Nov 9;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Borst P., Evers R., Kool M., Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000 Aug 16;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Bottero V., Busuttil V., Loubat A., Magné N., Fischel J. L., Milano G., Peyron J. F. Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res. 2001 Nov 1;61(21):7785–7791. [PubMed] [Google Scholar]
- Bradshaw D. M., Arceci R. J. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J Clin Oncol. 1998 Nov;16(11):3674–3690. doi: 10.1200/JCO.1998.16.11.3674. [DOI] [PubMed] [Google Scholar]
- Chen F., Castranova V., Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001 Aug;159(2):387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M., Sulli A., Pizzorni C., Seriolo B., Straub R. H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001 Aug;60(8):729–735. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001 Dec;33(6):475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- Djimdé A., Doumbo O. K., Cortese J. F., Kayentao K., Doumbo S., Diourté Y., Coulibaly D., Dicko A., Su X. Z., Nomura T. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001 Jan 25;344(4):257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Doyle L. A., Yang W., Abruzzo L. V., Krogmann T., Gao Y., Rishi A. K., Ross D. D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gadangi P., Longaker M., Naime D., Levin R. I., Recht P. A., Montesinos M. C., Buckley M. T., Carlin G., Cronstein B. N. The anti-inflammatory mechanism of sulfasalazine is related to adenosine release at inflamed sites. J Immunol. 1996 Mar 1;156(5):1937–1941. [PubMed] [Google Scholar]
- Galpin A. J., Schuetz J. D., Masson E., Yanishevski Y., Synold T. W., Barredo J. C., Pui C. H., Relling M. V., Evans W. E. Differences in folylpolyglutamate synthetase and dihydrofolate reductase expression in human B-lineage versus T-lineage leukemic lymphoblasts: mechanisms for lineage differences in methotrexate polyglutamylation and cytotoxicity. Mol Pharmacol. 1997 Jul;52(1):155–163. doi: 10.1124/mol.52.1.155. [DOI] [PubMed] [Google Scholar]
- Goekoop Y. P., Allaart C. F., Breedveld F. C., Dijkmans B. A. Combination therapy in rheumatoid arthritis. Curr Opin Rheumatol. 2001 May;13(3):177–183. doi: 10.1097/00002281-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Gottesman Michael M., Fojo Tito, Bates Susan E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002 Jan;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Hooijberg J. H., Broxterman H. J., Kool M., Assaraf Y. G., Peters G. J., Noordhuis P., Scheper R. J., Borst P., Pinedo H. M., Jansen G. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 1999 Jun 1;59(11):2532–2535. [PubMed] [Google Scholar]
- Huang T. T., Wuerzberger-Davis S. M., Seufzer B. J., Shumway S. D., Kurama T., Boothman D. A., Miyamoto S. NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J Biol Chem. 2000 Mar 31;275(13):9501–9509. doi: 10.1074/jbc.275.13.9501. [DOI] [PubMed] [Google Scholar]
- Jansen G., Mauritz R., Drori S., Sprecher H., Kathmann I., Bunni M., Priest D. G., Noordhuis P., Schornagel J. H., Pinedo H. M. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. J Biol Chem. 1998 Nov 13;273(46):30189–30198. doi: 10.1074/jbc.273.46.30189. [DOI] [PubMed] [Google Scholar]
- Jonker Johan W., Buitelaar Marije, Wagenaar Els, Van Der Valk Martin A., Scheffer George L., Scheper Rik J., Plosch Torsten, Kuipers Folkert, Elferink Ronald P. J. Oude, Rosing Hilde. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002 Nov 12;99(24):15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I., Sarkadi B., Váradi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999 Dec 6;1461(2):237–262. doi: 10.1016/s0005-2736(99)00161-3. [DOI] [PubMed] [Google Scholar]
- Landewé Robert B. M., Boers Maarten, Verhoeven Arco C., Westhovens Rene, van de Laar Mart A. F. J., Markusse Harry M., van Denderen J. Christiaan, Westedt Marie Louise, Peeters Andre J., Dijkmans Ben A. C. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002 Feb;46(2):347–356. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- Leslie E. M., Deeley R. G., Cole S. P. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001 Oct 5;167(1):3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- Liang E., Proudfoot J., Yazdanian M. Mechanisms of transport and structure-permeability relationship of sulfasalazine and its analogs in Caco-2 cell monolayers. Pharm Res. 2000 Oct;17(10):1168–1174. doi: 10.1023/a:1026450326712. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., van der Heijde D. M., St Clair E. W., Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Weisman M., Emery P., Feldmann M. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000 Nov 30;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Liptay S., Bachem M., Häcker G., Adler G., Debatin K. M., Schmid R. M. Inhibition of nuclear factor kappa B and induction of apoptosis in T-lymphocytes by sulfasalazine. Br J Pharmacol. 1999 Dec;128(7):1361–1369. doi: 10.1038/sj.bjp.0702937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman T., Druley T. E., Stein W. D., Bates S. E. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001 Jun;58(7):931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel A., Wong A., Strand V., Tugwell P., Wells G., Bombardier C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2000 Sep;39(9):975–981. doi: 10.1093/rheumatology/39.9.975. [DOI] [PubMed] [Google Scholar]
- Maliepaard M., Scheffer G. L., Faneyte I. F., van Gastelen M. A., Pijnenborg A. C., Schinkel A. H., van De Vijver M. J., Scheper R. J., Schellens J. H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001 Apr 15;61(8):3458–3464. [PubMed] [Google Scholar]
- Mauritz R., Bekkenk M. W., Rots M. G., Pieters R., Mini E., van Zantwijk C. H., Veerman A. J., Peters G. J., Jansen G. Ex vivo activity of methotrexate versus novel antifolate inhibitors of dihydrofolate reductase and thymidylate synthase against childhood leukemia cells. Clin Cancer Res. 1998 Oct;4(10):2399–2410. [PubMed] [Google Scholar]
- O'Dell James R., Leff Robert, Paulsen Gail, Haire Claire, Mallek Jack, Eckhoff P. James, Fernandez Ana, Blakely Kent, Wees Steven, Stoner Julie. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 May;46(5):1164–1170. doi: 10.1002/art.10228. [DOI] [PubMed] [Google Scholar]
- Robbiani D. F., Finch R. A., Jäger D., Muller W. A., Sartorelli A. C., Randolph G. J. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000 Nov 22;103(5):757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Rodenburg R. J., Ganga A., van Lent P. L., van de Putte L. B., van Venrooij W. J. The antiinflammatory drug sulfasalazine inhibits tumor necrosis factor alpha expression in macrophages by inducing apoptosis. Arthritis Rheum. 2000 Sep;43(9):1941–1950. doi: 10.1002/1529-0131(200009)43:9<1941::AID-ANR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ross D. D., Karp J. E., Chen T. T., Doyle L. A. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000 Jul 1;96(1):365–368. [PubMed] [Google Scholar]
- Ross D. D., Yang W., Abruzzo L. V., Dalton W. S., Schneider E., Lage H., Dietel M., Greenberger L., Cole S. P., Doyle L. A. Atypical multidrug resistance: breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. J Natl Cancer Inst. 1999 Mar 3;91(5):429–433. doi: 10.1093/jnci/91.5.429. [DOI] [PubMed] [Google Scholar]
- Rots M. G., Pieters R., Kaspers G. J., Veerman A. J., Peters G. J., Jansen G. Classification of ex vivo methotrexate resistance in acute lymphoblastic and myeloid leukaemia. Br J Haematol. 2000 Sep;110(4):791–800. doi: 10.1046/j.1365-2141.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- Scheffer G. L., Kool M., Heijn M., de Haas M., Pijnenborg A. C., Wijnholds J., van Helvoort A., de Jong M. C., Hooijberg J. H., Mol C. A. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res. 2000 Sep 15;60(18):5269–5277. [PubMed] [Google Scholar]
- Scheffer G. L., Maliepaard M., Pijnenborg A. C., van Gastelen M. A., de Jong M. C., Schroeijers A. B., van der Kolk D. M., Allen J. D., Ross D. D., van der Valk P. Breast cancer resistance protein is localized at the plasma membrane in mitoxantrone- and topotecan-resistant cell lines. Cancer Res. 2000 May 15;60(10):2589–2593. [PubMed] [Google Scholar]
- Scheffer G. L., Schroeijers A. B., Izquierdo M. A., Wiemer E. A., Scheper R. J. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Curr Opin Oncol. 2000 Nov;12(6):550–556. doi: 10.1097/00001622-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Smedegård G., Björk J. Sulphasalazine: mechanism of action in rheumatoid arthritis. Br J Rheumatol. 1995 Nov;34 (Suppl 2):7–15. [PubMed] [Google Scholar]
- Symmons D. P. Knee pain in older adults: the latest musculoskeletal "epidemic". Ann Rheum Dis. 2001 Feb;60(2):89–90. doi: 10.1136/ard.60.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P. P., Firestein G. S. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001 Jan;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Dalton W. S., Parrish P. R., Gleason M. C., Bellamy W. T., Thompson F. H., Roe D. J., Trent J. M. Different mechanisms of decreased drug accumulation in doxorubicin and mitoxantrone resistant variants of the MCF7 human breast cancer cell line. Br J Cancer. 1991 Jun;63(6):923–929. doi: 10.1038/bjc.1991.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. H., Liang Y. C., Lin-Shiau S. Y., Lin J. K. Suppression of TNFalpha-mediated NFkappaB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem. 1999 Sep 15;74(4):606–615. [PubMed] [Google Scholar]
- Ulfgren A. K., Andersson U., Engström M., Klareskog L., Maini R. N., Taylor P. C. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000 Nov;43(11):2391–2396. doi: 10.1002/1529-0131(200011)43:11<2391::AID-ANR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Volk E. L., Rohde K., Rhee M., McGuire J. J., Doyle L. A., Ross D. D., Schneider E. Methotrexate cross-resistance in a mitoxantrone-selected multidrug-resistant MCF7 breast cancer cell line is attributable to enhanced energy-dependent drug efflux. Cancer Res. 2000 Jul 1;60(13):3514–3521. [PubMed] [Google Scholar]
- Wahl C., Liptay S., Adler G., Schmid R. M. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998 Mar 1;101(5):1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. K., Liptay S., Wirth T., Adler G., Schmid R. M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000 Nov;119(5):1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillieres Clin Rheumatol. 1995 Nov;9(4):619–632. doi: 10.1016/s0950-3579(05)80305-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Gaynor R. B. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001 Jan;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Kuo M. T. NF-kappaB-mediated induction of mdr1b expression by insulin in rat hepatoma cells. J Biol Chem. 1997 Jun 13;272(24):15174–15183. doi: 10.1074/jbc.272.24.15174. [DOI] [PubMed] [Google Scholar]
- Zhou S., Schuetz J. D., Bunting K. D., Colapietro A. M., Sampath J., Morris J. J., Lagutina I., Grosveld G. C., Osawa M., Nakauchi H. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001 Sep;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- de Bruin M., Miyake K., Litman T., Robey R., Bates S. E. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999 Nov 15;146(2):117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- van der Heijden J., de Jong M. C., Dijkmans B. A. C., Lems W. F., Oerlemans R., Kathmann I., Scheffer G. L., Scheper R. J., Assaraf Y. G., Jansen G. Acquired resistance of human T cells to sulfasalazine: stability of the resistant phenotype and sensitivity to non-related DMARDs. Ann Rheum Dis. 2004 Feb;63(2):131–137. doi: 10.1136/ard.2003.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk Dorina M., Vellenga Edo, Scheffer George L., Müller Michael, Bates Susan E., Scheper Rik J., de Vries Elisabeth G. E. Expression and activity of breast cancer resistance protein (BCRP) in de novo and relapsed acute myeloid leukemia. Blood. 2002 May 15;99(10):3763–3770. doi: 10.1182/blood.v99.10.3763. [DOI] [PubMed] [Google Scholar]