Abstract
Objective: To determine whether 17ß-oestradiol (E2) modulates interleukin (IL) 1ß-induced proteoglycan degradation in chondrocytes, and to analyse the part played by metalloproteinases (MMPs) in this process.
Methods: Primary cultured rabbit articular chondrocytes were prepared and treated with 10 ng/ml IL1ß combined or not with 0.1–10 nM E2. Neosynthesised proteoglycans (PGs) were evaluated after incorporation of [35SO4]sulphate and further analysed after chromatography on a Sepharose 2B column. Chondrocyte mRNA levels of aggrecan, MMP-1, -3, -13, and tissue inhibitor of metalloproteinase-1 (TIMP-1) were studied by northern blot. MMP-1 activity was measured by zymography. MMP-1 gene transcription was studied by transient transfection of chondrocytes with an MMP-1-luciferase construct.
Results: E2 modulated the IL1ß-induced total sulphated PGs in rabbit articular chondrocytes, which decreased as the E2 concentration was increased. At a low concentration (0.1 nmol/l) E2 counteracts the IL1ß-induced decrease in sulphated PG, while at high concentration (10 nmol/l) E2 enhances the IL1ß effects. A biphasic E2 effect was also observed on IL1ß-induced disaggregation of PG, 53–58 kDa gelatinolytic activity, and MMP-1, -3, and -13 mRNA levels. In contrast, E2 did not modify the level of aggrecan mRNA and had no effect on TIMP-1 mRNA expression. Finally, simultaneous addition of IL1ß and E2 (0.1–10 nmol/l) did not modify IL1ß-induced MMP-1-luciferase activity, suggesting that E2 effects probably occur at the post-transcriptional level of MMP gene expression.
Conclusion: Oestrogen concentration may have an inverse effect on IL1ß stimulated proteoglycan degradation and MMP production by chondrocytes.
Full Text
The Full Text of this article is available as a PDF (472.4 KB).
Figure 1 .
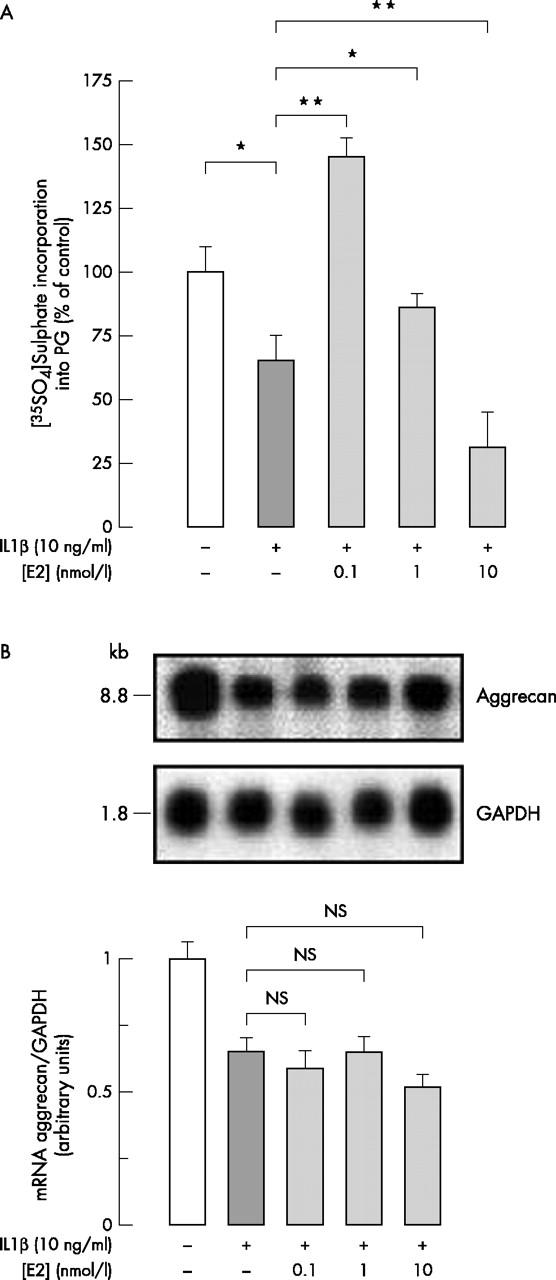
(A) [35SO4]Sulphate incorporation into PGs synthesised and secreted by chondrocytes treated or not with 10 ng/ml IL1ß combined or not with E2 (0.1–10 nmol/l). Articular chondrocytes were cultured until confluency. After 24 hours without FCS, cells were incubated with 1.5 µCi/ml [35SO4]sulphate for 20 hours with or without effectors. [35SO4]Sulphated proteoglycans were extracted as described in "Materials and methods". In each experiment six similarly treated flasks were prepared, three being used for the sulphation assay and the other three for DNA measurement. Radioactivity incorporated into [35SO4]sulphated PG was calculated as mean (SD) dpm/10 µg DNA from four experiments performed with different animal donors and expressed as a percentage of controls incubated without effectors (*p⩽0.05; **p<0.01). (B) Northern blot showing aggrecan mRNA hybridisation signal in chondrocytes treated or not with 10 ng/ml IL1ß combined or not with E2 (0.1–10 nmol/l). Total mRNA was extracted after 20 hours' incubation with or without effectors. The upper part shows the hybridisation signal to the rabbit aggrecan probe and to a human GAPDH probe which indicates the relative amount of RNA loaded in each lane. These data are representative of one of three independent experiments. The lower part shows the densitometric quantification of the autoradiogram expressed as the aggrecan/GAPDH band density ratio. Results are the mean (SD) of three experiments performed with different animal donors.
Figure 2 .
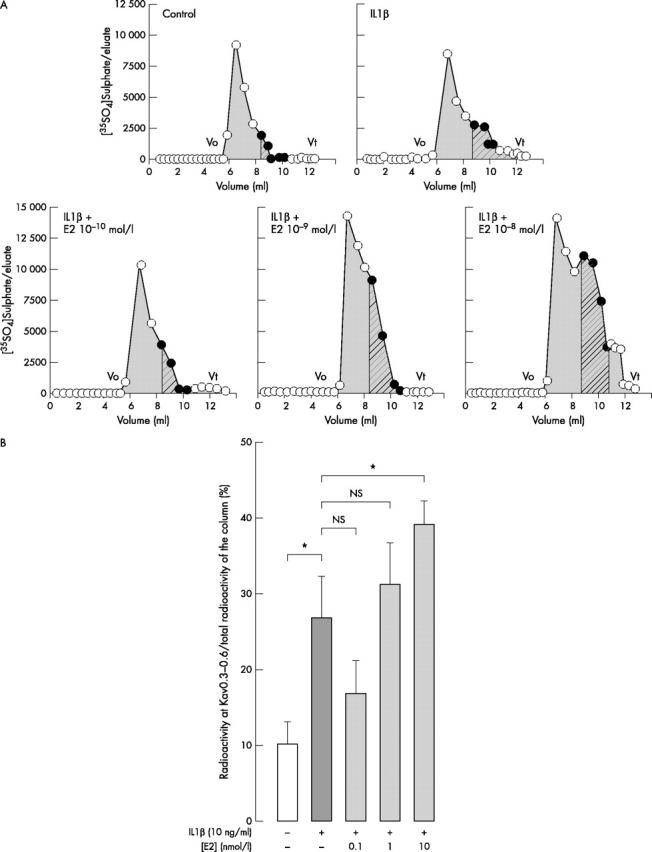
(A) Sepharose 2B elution profile of [35SO4]sulphated proteoglycans synthesised and secreted by chondrocytes treated or not with IL1ß combined or not with E2 (0.1–10 nmol/l). Two similarly treated flasks were prepared in each experiment. Confluent chondrocytes were incubated with [35SO4]sulphate for 20 hours with or without effectors as described in fig 1. [35SO4]Sulphated PGs were extracted in associative conditions with protease inhibitors as described in "Materials and methods". Aliquots were applied to a column of Sepharose 2B. Eluates were collected and the radioactivity measured by scintillation counting. Vo is the void volume and Vt is the total volume of the column. In each experimental condition, LMW radioactive material (hatched grey) eluted between Kav 0.3 and 0.6, is expressed as a percentage of total radioactivity eluted on the column between Vo and Vt (plain grey). The yield of the column is >90%. Data are representative of a series of flasks examined in one of three independent experiments. (B). For each column, radioactivity eluted at Kav 0.3–0.6 (LMW PG) was measured and expressed as a percentage of the total radioactivity eluted on the column (Vo to Vt). In each experiment two similarly treated flasks were analysed. Results are expressed as the mean (SD) of three experiments performed with different animal donors (*p⩽0.05).
Figure 3 .
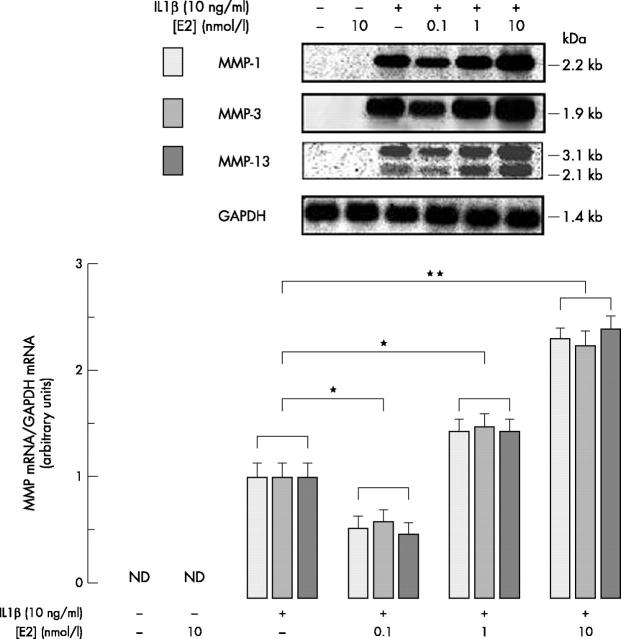
Northern blot showing the effects of E2 on IL1ß-induced MMP-1, -3, and -13 mRNA hybridisation signals. Confluent chondrocytes were incubated for 20 hours with or without effectors as described above. After 20 hours, total RNA was extracted and analysed as in fig 1B. The upper part shows the hybridisation signal to the rabbit MMP-1, -3, -13 probes and to the human GAPDH probe. Data are representative of one of four independent experiments. The lower part shows the densitometric quantification of the autoradiogram expressed as the MMP/GAPDH band density ratio. Data are the mean (SD) from four independent experiments (*p⩽0.05, **p<0.01).
Figure 4 .
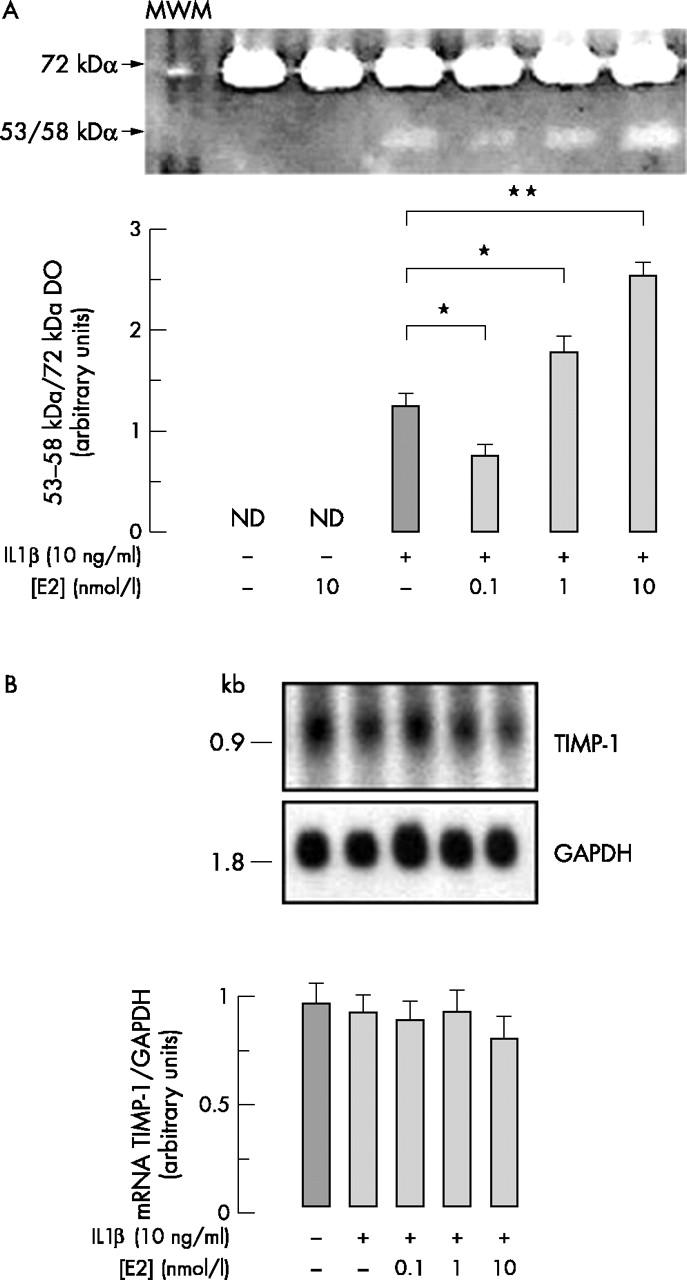
(A) Gelatinolytic activities and (B) TIMP-1 mRNA levels in chondrocytes treated or not with 10 ng/ml IL1ß combined or not with E2 (0.1–10 nmol/l). Confluent chondrocytes were incubated for 20 hours with or without effectors as described in fig 1. Culture medium was collected and subjected to gelatin zymography as described in "Materials and methods". Total RNA was extracted from the corresponding cultured flasks and analysed as in fig 1B for TIMP-1 hybridisation signal. (A) Upper part: the gelatinolytic bands are representative of a series of flasks from one of four independent experiments. Lower part: the level of gelatinolytic bands was determined by scanning video-densitometry. Because the 72 kDa band densitometry was not modified by IL1ß or E2, this band was used as internal control. The densitometric quantification of the IL1ß-induced 53–58 kDa band was thus expressed as the 53–58 kDa/72 kDa band ratio. Data are the mean (SD) of results obtained from four independent experiments performed with different animals. (B) Northern blot analysis of TIMP-1 mRNA hybridisation signal. The upper part shows the hybridisation signal to the rabbit TIMP-1 probe and to a human GAPDH probe. Data are representative of a series of flasks from one of four independent experiments. The lower part shows the densitometric quantification of the autoradiogram expressed as mean (SD) of TIMP-1/GAPDH band density ratio measured in four independent experiments.
Figure 5 .
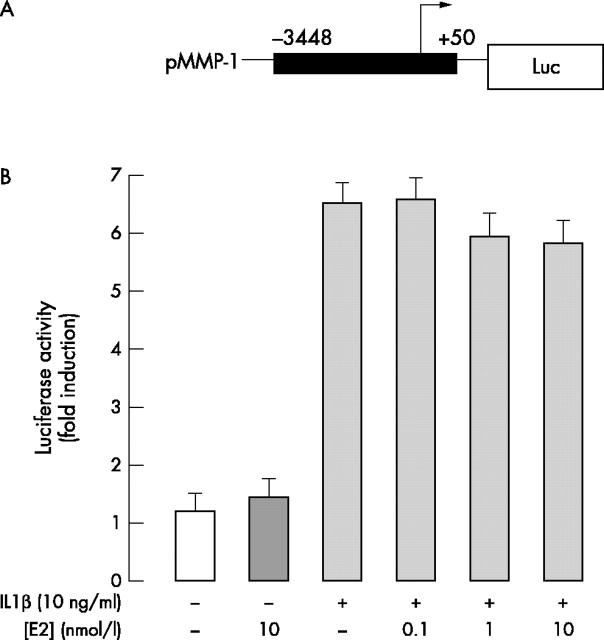
MMP-1 gene promoter activity. The upper part represents the design of the 3.45 kb of the 5' flanking region linked to the luciferase reported gene used in DNA transfection experiments. Chondrocytes were transiently cotransfected with MMP-1 gene promoter and with a ß-galactosidase expression vector and further treated with each effector for 20 hours. Each point was measured in triplicate. The lower part shows the relative luciferase activity calculated as fold induction in treated cells with respect to the basal activity measured in untreated cells. Values for the luciferase activity were normalised to the ß-galactosidase activity. Results are the mean (SD) of three independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arao Yukitomo, Kikuchi Atsumi, Ikeda Kazuhiro, Nomoto Satoshi, Horiguchi Hyogo, Kayama Fujio. A+U-rich-element RNA-binding factor 1/heterogeneous nuclear ribonucleoprotein D gene expression is regulated by oestrogen in the rat uterus. Biochem J. 2002 Jan 1;361(Pt 1):125–132. doi: 10.1042/0264-6021:3610125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelle A., Gardell S., Heinegård D. Proteoglycans of articular cartilage from bovine lower femoral epiphysis. Extraction and characterisation of proteoglycans from two sites within the same joint. Connect Tissue Res. 1974;2(2):111–116. doi: 10.3109/03008207409152096. [DOI] [PubMed] [Google Scholar]
- Blanchard O., Tsagris L., Rappaport R., Duval-Beaupere G., Corvol M. Age-dependent responsiveness of rabbit and human cartilage cells to sex steroids in vitro. J Steroid Biochem Mol Biol. 1991;40(4-6):711–716. doi: 10.1016/0960-0760(91)90295-g. [DOI] [PubMed] [Google Scholar]
- Caterson B., Flannery C. R., Hughes C. E., Little C. B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000 Aug;19(4):333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corvol M. T., Carrascosa A., Tsagris L., Blanchard O., Rappaport R. Evidence for a direct in vitro action of sex steroids on rabbit cartilage cells during skeletal growth: influence of age and sex. Endocrinology. 1987 Apr;120(4):1422–1429. doi: 10.1210/endo-120-4-1422. [DOI] [PubMed] [Google Scholar]
- Corvol M. T., Dumontier M. F., Rappaport R. Culture of chondrocytes from the proliferative zone of epiphyseal growth plate cartilage from prepubertal rabbits. Biomedicine. 1975 Apr 10;23(3):103–107. [PubMed] [Google Scholar]
- Dayani N., Corvol M. T., Robel P., Eychenne B., Moncharmont B., Tsagris L., Rappaport R. Estrogen receptors in cultured rabbit articular chondrocytes: influence of age. J Steroid Biochem. 1988 Sep;31(3):351–356. doi: 10.1016/0022-4731(88)90361-5. [DOI] [PubMed] [Google Scholar]
- Deng S. J., Bickett D. M., Mitchell J. L., Lambert M. H., Blackburn R. K., Carter H. L., 3rd, Neugebauer J., Pahel G., Weiner M. P., Moss M. L. Substrate specificity of human collagenase 3 assessed using a phage-displayed peptide library. J Biol Chem. 2000 Oct 6;275(40):31422–31427. doi: 10.1074/jbc.M004538200. [DOI] [PubMed] [Google Scholar]
- Dodson R. E., Shapiro D. J. Vigilin, a ubiquitous protein with 14 K homology domains, is the estrogen-inducible vitellogenin mRNA 3'-untranslated region-binding protein. J Biol Chem. 1997 May 9;272(19):12249–12252. doi: 10.1074/jbc.272.19.12249. [DOI] [PubMed] [Google Scholar]
- Fernihough J. K., Richmond R. S., Carlson C. S., Cherpes T., Holly J. M., Loeser R. F. Estrogen replacement therapy modulation of the insulin-like growth factor system in monkey knee joints. Arthritis Rheum. 1999 Oct;42(10):2103–2111. doi: 10.1002/1529-0131(199910)42:10<2103::AID-ANR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Fosang A. J., Last K., Knäuper V., Murphy G., Neame P. J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996 Feb 12;380(1-2):17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- Guccione Michael, Silbiger Sharon, Lei Jun, Neugarten Joel. Estradiol upregulates mesangial cell MMP-2 activity via the transcription factor AP-2. Am J Physiol Renal Physiol. 2002 Jan;282(1):F164–F169. doi: 10.1152/ajprenal.0318.2000. [DOI] [PubMed] [Google Scholar]
- Hall J. M., McDonnell D. P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999 Dec;140(12):5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Ham Kimberley D., Loeser Richard F., Lindgren Bruce R., Carlson Cathy S. Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum. 2002 Jul;46(7):1956–1964. doi: 10.1002/art.10406. [DOI] [PubMed] [Google Scholar]
- Hellio Le Graverand M. P., Reno C., Hart D. A. Influence of pregnancy on gene expression in rabbit articular cartilage. Osteoarthritis Cartilage. 1998 Sep;6(5):341–350. doi: 10.1053/joca.1998.0133. [DOI] [PubMed] [Google Scholar]
- Kapila S., Xie Y. Targeted induction of collagenase and stromelysin by relaxin in unprimed and beta-estradiol-primed diarthrodial joint fibrocartilaginous cells but not in synoviocytes. Lab Invest. 1998 Aug;78(8):925–938. [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Larsson S. E., Kuettner K. E. Microchemical studies of acid glycosaminoglycans from isolated chondrocytes in suspension. Calcif Tissue Res. 1974;14(1):49–58. doi: 10.1007/BF02060282. [DOI] [PubMed] [Google Scholar]
- Liao E. Y., Luo X. H. Effects of 17beta-estradiol on the expression of matrix metalloproteinase-1, -2 and tissue inhibitor of metalloproteinase-1 in human osteoblast-like cell cultures. Endocrine. 2001 Aug;15(3):291–295. doi: 10.1385/ENDO:15:3:291. [DOI] [PubMed] [Google Scholar]
- Little Christopher B., Hughes Clare E., Curtis Clare L., Janusz Mike J., Bohne Richard, Wang-Weigand Sherry, Taiwo Yetunde O., Mitchell Peter G., Otterness Ivan G., Flannery Carl R. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002 Apr;21(3):271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 1999 Jul;7(4):371–373. doi: 10.1053/joca.1998.0214. [DOI] [PubMed] [Google Scholar]
- McKenna N. J., Lanz R. B., O'Malley B. W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999 Jun;20(3):321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Mengshol John A., Mix Kimberlee S., Brinckerhoff Constance E. Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 2002 Jan;46(1):13–20. doi: 10.1002/1529-0131(200201)46:1<13::aid-art497>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Billington C. J. Articular cartilage and changes in arthritis: matrix degradation. Arthritis Res. 2001 Sep 6;3(6):337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M. C., Harper R. P., Le C. T., Wong B. S. Effects of estrogen on the condylar cartilage of the rat mandible in organ culture. J Oral Maxillofac Surg. 1999 Jul;57(7):818–823. doi: 10.1016/s0278-2391(99)90823-6. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Fiacco G. J., Walling H. W., Barmina O. Y., Jeffrey J. J., Ruh M. F. Effects of dioxin and estrogen on collagenase-3 in UMR 106-01 osteosarcoma cells. Arch Biochem Biophys. 2000 Oct 15;382(2):182–188. doi: 10.1006/abbi.2000.1992. [DOI] [PubMed] [Google Scholar]
- Pastori R. L., Moskaitis J. E., Buzek S. W., Schoenberg D. R. Coordinate estrogen-regulated instability of serum protein-coding messenger RNAs in Xenopus laevis. Mol Endocrinol. 1991 Apr;5(4):461–468. doi: 10.1210/mend-5-4-461. [DOI] [PubMed] [Google Scholar]
- Potier M., Elliot S. J., Tack I., Lenz O., Striker G. E., Striker L. J., Karl M. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001 Feb;12(2):241–251. doi: 10.1681/ASN.V122241. [DOI] [PubMed] [Google Scholar]
- Rajabi M., Solomon S., Poole A. R. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology. 1991 Feb;128(2):863–871. doi: 10.1210/endo-128-2-863. [DOI] [PubMed] [Google Scholar]
- Rannou F., Corvol M. T., Hudry C., Anract P., Dumontier M. F., Tsagris L., Revel M., Poiraudeau S. Sensitivity of anulus fibrosus cells to interleukin 1 beta. Comparison with articular chondrocytes. Spine (Phila Pa 1976) 2000 Jan;25(1):17–23. doi: 10.1097/00007632-200001010-00005. [DOI] [PubMed] [Google Scholar]
- Richmond R. S., Carlson C. S., Register T. C., Shanker G., Loeser R. F. Functional estrogen receptors in adult articular cartilage: estrogen replacement therapy increases chondrocyte synthesis of proteoglycans and insulin-like growth factor binding protein 2. Arthritis Rheum. 2000 Sep;43(9):2081–2090. doi: 10.1002/1529-0131(200009)43:9<2081::AID-ANR20>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Schwartz N. B., Pirok E. W., 3rd, Mensch J. R., Jr, Domowicz M. S. Domain organization, genomic structure, evolution, and regulation of expression of the aggrecan gene family. Prog Nucleic Acid Res Mol Biol. 1999;62:177–225. doi: 10.1016/s0079-6603(08)60508-5. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Turner A. S., Athanasiou K. A., Zhu C. F., Alvis M. R., Bryant H. U. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthritis Cartilage. 1997 Jan;5(1):63–69. doi: 10.1016/s1063-4584(97)80032-5. [DOI] [PubMed] [Google Scholar]
- Ushiyama T., Ueyama H., Inoue K., Ohkubo I., Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999 Nov;7(6):560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- Vincenti M. P., Coon C. I., Brinckerhoff C. E. Nuclear factor kappaB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1beta-stimulated synovial fibroblasts. Arthritis Rheum. 1998 Nov;41(11):1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wluka A. E., Cicuttini F. M., Spector T. D. Menopause, oestrogens and arthritis. Maturitas. 2000 Jun 30;35(3):183–199. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]