Abstract
Background: Expression of signal transducer and activator of transcription 1 (STAT1), the mediator of interferon (IFN) signalling, is raised in synovial tissue (ST) from patients with rheumatoid arthritis (RA).
Objectives: To determine the extent to which this pathway is activated by phosphorylation in RA synovium. Additionally, to investigate the cellular basis of STAT1 activation in RA ST.
Methods: ST specimens from 12 patients with RA and 14 disease controls (patients with osteoarthritis and reactive arthritis) were analysed by immunohistochemistry, using antibodies to STAT1, tyrosine phosphorylated STAT1, and serine phosphorylated STAT1. Lysates of cultured fibroblast-like synoviocytes stimulated with IFNß were analysed by western blotting. Phenotypic characterisation of cells expressing STAT1 in RA ST was performed by double immunolabelling for STAT1 and CD3, CD22, CD55, or CD68.
Results: Raised levels of total STAT1 protein and both its activated tyrosine and serine phosphorylated forms were seen in RA synovium as compared with controls. STAT1 was predominantly abundant in T and B lymphocytes in focal inflammatory infiltrates and in fibroblast-like synoviocytes in the intimal lining layer. Raised levels of STAT1 are sustained in cultured RA compared with OA fibroblast-like synoviocytes, and STAT1 serine and tyrosine phosphorylation is rapidly induced upon stimulation with IFNß.
Conclusion: These results demonstrate activation of the STAT1 pathway in RA synovium by raised STAT1 protein expression and concomitantly increased tyrosine (701) and serine (727) phosphorylation. High expression of STAT1 is intrinsic to RA fibroblast-like synoviocytes in the intimal lining layer, whereas activation of the pathway by phosphorylation is an active process.
Full Text
The Full Text of this article is available as a PDF (1,023.6 KB).
Figure 1 .
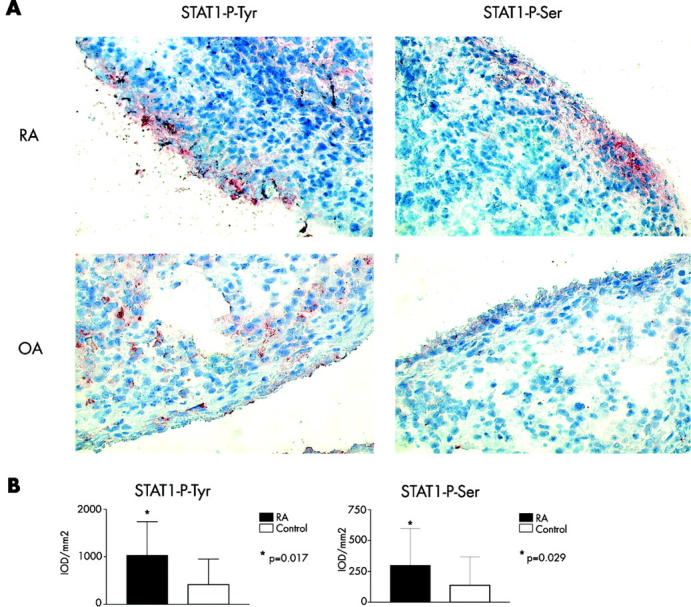
Increased STAT1 phosphorylation on tyrosine and serine in RA ST as compared with controls. Serial ST sections from 12 patients with RA and 14 controls were stained for STAT1-phospho-tyrosine and STAT1-phospho-serine using aminoethylcarbazole as dye (A) representative sections are shown. Counterstaining was performed using Mayer's haematoxylin. (Original magnification x400.) Sections were analysed using computer assisted digital image analysis (B). Results are shown as means, errors bars indicate SD; on the y axis the mean IOD per mm2 of tissue (see "Materials and methods") is shown.
Figure 2 .
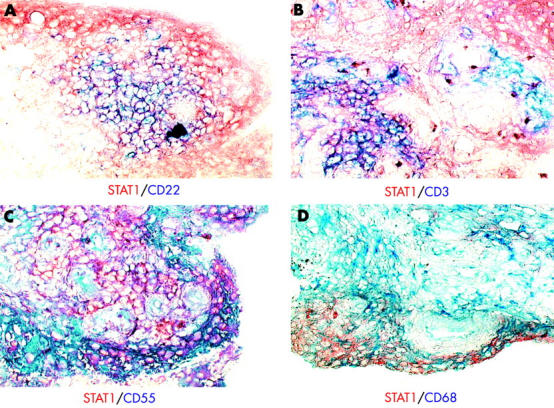
Immunohistochemical double labelling of the ST of four patients with RA (table 1; patients Nos 4, 5, 7, and 13; representative sections are shown) for STAT1 and CD22+ B cells (A), CD3+ T cells (B), CD55+ fibroblast-like synoviocytes (C), or CD68+ macrophages (D). STAT1 was detected using aminoethylcarbazole as dye (red) and CD molecules were detected using AP substrate (blue); no counterstaining was performed (see "Materials and methods"). (Original magnificationx400.)
Figure 3 .
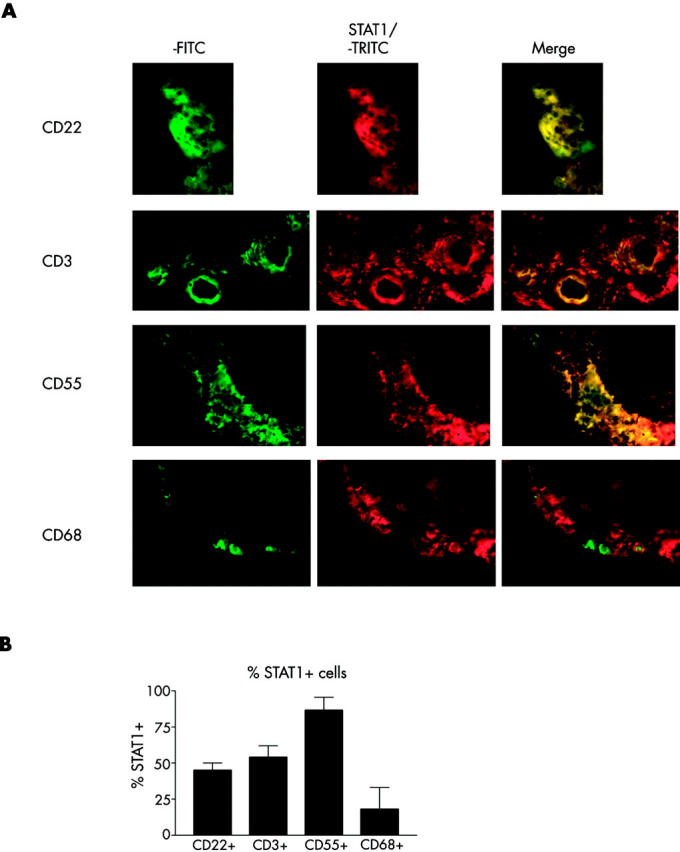
Double immunofluorescence was performed on the ST of four patients with RA (table 1; patients Nos 1, 2, 3, and 10). (A) STAT1 (tetramethylrhodamine) and CD22+ B cells, CD3+ T cells, CD55+ fibroblast-like synoviocytes, or CD68+ macrophages (fluorescein). No counterstaining was performed. (Original magnification x400.) (B) Percentage STAT1+ cells of, respectively, total CD22+, CD3+, CD55+, or CD68+ cells. Bars represent mean (SD) (n = 4).
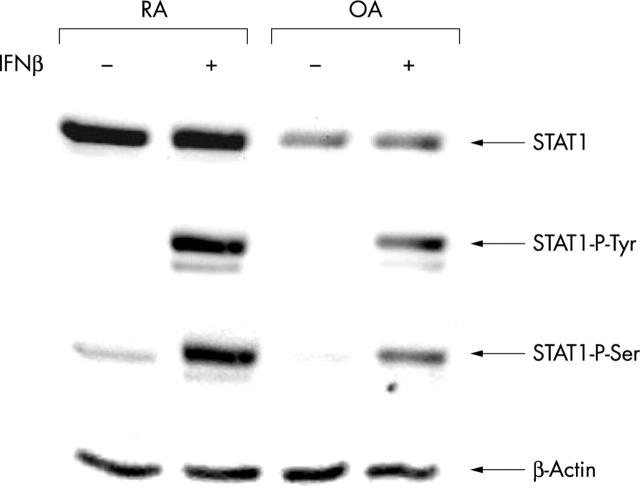
Figure 4 .
STAT1 expression is raised in RA compared with OA fibroblast-like synoviocytes (top panel) and STAT1 tyrosine and serine phosphorylation are induced by stimulation with IFNß (second and third panels). Fourth passage RA and OA synoviocytes were treated with IFNß (250 U/ml). Cell extracts were prepared 30 minutes after cytokine stimulation, corrected for total protein content, and analysed by immunoblotting.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Alarcón G., Appelrouth D., Bloch D., Borenstein D., Brandt K., Brown C., Cooke T. D., Daniel W., Feldman D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991 May;34(5):505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- Andreakos Evangelos T., Foxwell Brian M., Brennan Fionula M., Maini Ravinder N., Feldmann Marc. Cytokines and anti-cytokine biologicals in autoimmunity: present and future. Cytokine Growth Factor Rev. 2002 Aug-Oct;13(4-5):299–313. doi: 10.1016/s1359-6101(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bromberg J. F., Horvath C. M., Wen Z., Schreiber R. D., Darnell J. E., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J., Darnell J. E., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000 May 15;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Chin Y. E., Kitagawa M., Kuida K., Flavell R. A., Fu X. Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997 Sep;17(9):5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000 May 15;19(21):2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- Decker T., Kovarik P. Transcription factor activity of STAT proteins: structural requirements and regulation by phosphorylation and interacting proteins. Cell Mol Life Sci. 1999 Sep;55(12):1535–1546. doi: 10.1007/s000180050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca J. E., Edwards J. C. W., Blades S., Goulding N. J. Macrophage subpopulations in rheumatoid synovium: reduced CD163 expression in CD4+ T lymphocyte-rich microenvironments. Arthritis Rheum. 2002 May;46(5):1210–1216. doi: 10.1002/art.10207. [DOI] [PubMed] [Google Scholar]
- Hu Xiaoyu, Herrero Carmen, Li Wai-Ping, Antoniv Taras T., Falck-Pedersen Erik, Koch Alisa E., Woods James M., Haines G. Kenneth, Ivashkiv Lionel B. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002 Aug 12;3(9):859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- Kisseleva T., Bhattacharya S., Braunstein J., Schindler C. W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002 Feb 20;285(1-2):1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Haringman J. J., Ahern M. J., Breedveld F. C., Smith M. D., Tak P. P. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology (Oxford) 2000 Jan;39(1):43–49. doi: 10.1093/rheumatology/39.1.43. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., Smith M. D., Weedon H., Ahern M. J., Breedveld F. C., Tak P. P. Measurement of cytokine and adhesion molecule expression in synovial tissue by digital image analysis. Ann Rheum Dis. 2001 Mar;60(3):296–298. doi: 10.1136/ard.60.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan Maarten C., Reece Richard J., Smeets Tom J. M., Veale Douglas J., Emery Paul, Tak Paul P. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: Implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002 Aug;46(8):2034–2038. doi: 10.1002/art.10556. [DOI] [PubMed] [Google Scholar]
- Krause Anja, Scaletta Nicholas, Ji Jong-Dae, Ivashkiv Lionel B. Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol. 2002 Dec 1;169(11):6610–6616. doi: 10.4049/jimmunol.169.11.6610. [DOI] [PubMed] [Google Scholar]
- Kumar A., Commane M., Flickinger T. W., Horvath C. M., Stark G. R. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997 Nov 28;278(5343):1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Anderson E., Madani K., Rosen G. D. Loss of STAT1 expression confers resistance to IFN-gamma-induced apoptosis in ME180 cells. FEBS Lett. 1999 Oct 15;459(3):323–326. doi: 10.1016/s0014-5793(99)01283-1. [DOI] [PubMed] [Google Scholar]
- Lehtonen A., Matikainen S., Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol. 1997 Jul 15;159(2):794–803. [PubMed] [Google Scholar]
- O'Shea John J., Gadina Massimo, Schreiber Robert D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002 Apr;109 (Suppl):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Pap T., Müller-Ladner U., Gay R. E., Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000 Jun 8;2(5):361–367. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D., Akbar A. N., Girdlestone J., Orteu C. H., Borthwick N. J., Amft N., Scheel-Toellner D., Buckley C. D., Salmon M. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999 Mar;29(3):1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shankaran V., Ikeda H., Bruce A. T., White J. M., Swanson P. E., Old L. J., Schreiber R. D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001 Apr 26;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shen Y., Devgan G., Darnell J. E., Jr, Bromberg J. F. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A. 2001 Feb 6;98(4):1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouda T., Yoshida T., Hanada T., Wakioka T., Oishi M., Miyoshi K., Komiya S., Kosai K., Hanakawa Y., Hashimoto K. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001 Dec;108(12):1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T. J., Dolhain R. J., Breedveld F. C., Tak P. P. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. J Pathol. 1998 Sep;186(1):75–81. doi: 10.1002/(SICI)1096-9896(199809)186:1<75::AID-PATH142>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Tak P. P., van der Lubbe P. A., Cauli A., Daha M. R., Smeets T. J., Kluin P. M., Meinders A. E., Yanni G., Panayi G. S., Breedveld F. C. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995 Oct;38(10):1457–1465. doi: 10.1002/art.1780381012. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y., Firestein G. S. Pathogenesis of rheumatoid arthritis: the role of synoviocytes. Rheum Dis Clin North Am. 2001 May;27(2):355–371. doi: 10.1016/s0889-857x(05)70206-4. [DOI] [PubMed] [Google Scholar]
- Yokota A., Narazaki M., Shima Y., Murata N., Tanaka T., Suemura M., Yoshizaki K., Fujiwara H., Tsuyuguchi I., Kishimoto T. Preferential and persistent activation of the STAT1 pathway in rheumatoid synovial fluid cells. J Rheumatol. 2001 Sep;28(9):1952–1959. [PubMed] [Google Scholar]
- Youssef P. P., Triantafillou S., Parker A., Coleman M., Roberts-Thomson P. J., Ahern M. J., Smith M. D. Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J Rheumatol. 1997 Dec;24(12):2291–2298. [PubMed] [Google Scholar]
- van der Pouw Kraan T. C. T. M., van Gaalen F. A., Huizinga T. W. J., Pieterman E., Breedveld F. C., Verweij C. L. Discovery of distinctive gene expression profiles in rheumatoid synovium using cDNA microarray technology: evidence for the existence of multiple pathways of tissue destruction and repair. Genes Immun. 2003 Apr;4(3):187–196. doi: 10.1038/sj.gene.6363975. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan Tineke C. T. M., van Gaalen Floris A., Kasperkovitz Pia V., Verbeet Nicolette L., Smeets Tom J. M., Kraan Maarten C., Fero Mike, Tak Paul-Peter, Huizinga Tom W. J., Pieterman Elsbet. Rheumatoid arthritis is a heterogeneous disease: evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. 2003 Aug;48(8):2132–2145. doi: 10.1002/art.11096. [DOI] [PubMed] [Google Scholar]