Abstract
Objective: To analyse the distribution patterns of tenascin and proteoglycans in normal and osteoarthritic cartilage, and to determine the effect of interleukin 1ß (IL1ß) on aggrecan and tenascin expression by human articular chondrocytes in vitro.
Methods: Normal and osteoarthritic cartilage and bone samples were obtained during total knee replacements or necropsies. After fixation and decalcification, paraffin embedded specimens were sectioned perpendicular to the surface. Specimens were graded according to Mankin and subdivided into those with normal, and mild, moderate, and severe osteoarthritic lesions. Serial sections were immunostained for tenascin. Tenascin expression by healthy and osteoarthritic chondrocytes was quantified by real time polymerase chain reaction (PCR). Furthermore, in cell culture experiments, human articular chondrocytes were treated with 0.1 or 10 ng/ml IL1ß. Real time PCR analyses of aggrecan and tenascin transcripts (normalised 18S rRNA) were conducted to determine the effect of IL1ß on later mRNA levels.
Results: Tenascin was immunodetected in normal and osteoarthritic cartilage. In osteoarthritic cartilage increased tenascin staining was found. Tenascin was found specifically in upper OA cartilage showing a strong reduction of proteoglycans. Greatly increased tenascin transcript levels were detected in osteoarthritic cartilage compared with healthy articular cartilage. IL1ß treatment of articular chondrocytes in vitro significantly increased tenascin transcripts (~200% of control) and strongly reduced aggrecan mRNA levels (~42% of control).
Conclusions: During progression of osteoarthritis the switch in matrix synthesis occurs mainly in upper osteoarthritic cartilage. Furthermore, changes in synthesis patterns of osteoarthritic chondrocytes may be significantly influenced by IL1ß, probably diffusing from the joint cavity within the upper osteoarthritic cartilage.
Full Text
The Full Text of this article is available as a PDF (535.5 KB).
Figure 1 .
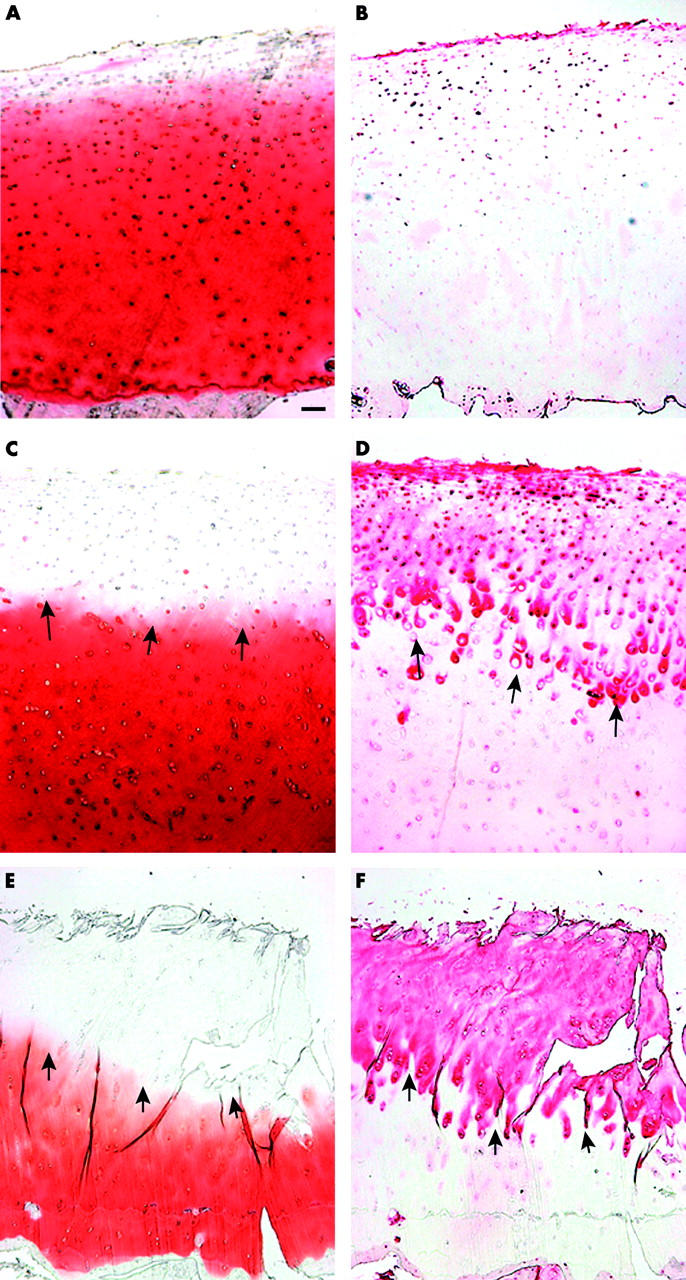
Serial sections of normal and OA cartilage. The tenascin deposition in healthy cartilage varied interindividually. Normal cartilage stained for proteoglycans (A) and immunostained for tenascin (B) shows pericellular/territorial tenascin deposition around the superficial and upper middle zone chondrocytes (weak immunostaining). (C) Mild OA lesion with decreased proteoglycan staining of the upper cartilage (safranin O staining). (D) Immunohistochemical analysis of tenascin in the same section as shown in C (note the territorial staining around the middle zone chondrocytes indicated by arrows). (E) Severe OA cartilage lesion showing a strong reduction of proteoglycans, deep clefts within the deep zone, and chondrocyte clusters in all zones (safranin O staining). (F) Immunostaining of tenascin in the same section as shown in E (note the strong tenascin staining in cartilage areas showing the absence of proteoglycans indicated by arrows). The bar in A represents 100 µm in A-F.
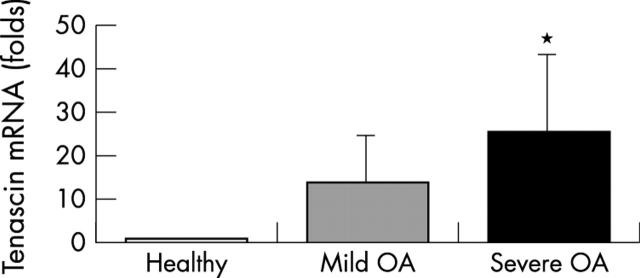
Figure 2 .
Bars represent the several-fold changes of tenascin mRNA levels (normalised to 18S rRNA) in mild and severe OA lesions compared with healthy cartilage samples (one fold expression). Bars are means (SD). *p<0.05 versus healthy samples.
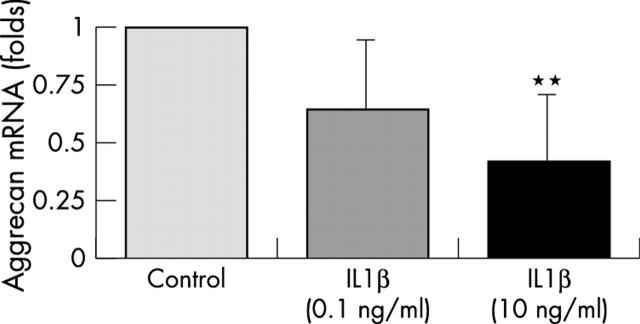
Figure 3 .
Bars represent the several-fold changes of aggrecan mRNA levels (normalised to 18S rRNA) of IL1ß treated chondrocytes compared with untreated control chondrocytes (one fold expression). Articular chondrocytes were cultured under serum free conditions for 18 hours in the absence or presence of 0.1 and 10 ng/ml IL1ß, respectively. Results are from four independent cell culture experiments. Bars represent means (SD). **p<0.01 versus control.
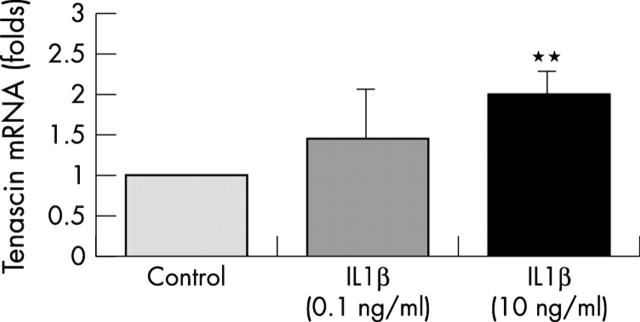
Figure 4 .
Bars represent the several-fold changes of tenascin mRNA levels (normalised to 18S rRNA) of IL1ß treated chondrocytes compared with untreated control chondrocytes (one fold expression). Articular chondrocytes were cultured under serum free conditions for 18 hours in the absence or presence of 0.1 and 10 ng/ml IL1ß, respectively. Results are from four independent experiments. Bars represent means (SD). **p<0.01 versus control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T., Vornehm S. I., Zeiler G., Dudhia J., von der Mark K., Bayliss M. T. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 1997 Mar;40(3):562–569. doi: 10.1002/art.1780400323. [DOI] [PubMed] [Google Scholar]
- Aigner T., Zien A., Gehrsitz A., Gebhard P. M., McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001 Dec;44(12):2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Chevalier X., Claudepierre P., Groult N., Godeau G. J. Influence of interleukin 1 beta on tenascin distribution in human normal and osteoarthritic cartilage: a quantitative immunohistochemical study. Ann Rheum Dis. 1996 Oct;55(10):772–775. doi: 10.1136/ard.55.10.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X., Groult N., Larget-Piet B., Zardi L., Hornebeck W. Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1994 Jul;37(7):1013–1022. doi: 10.1002/art.1780370706. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. L., Brown M., Ali S. Y., Brown R. A. An immunohistochemical study of fibronectin in human osteoarthritic and disease free articular cartilage. Ann Rheum Dis. 1987 Nov;46(11):809–815. doi: 10.1136/ard.46.11.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Moos V., Fickert S., Müller B., Weber U., Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999 Apr;26(4):870–879. [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Noda N., Minoura H., Nishiura R., Toyoda N., Imanaka-Yoshida K., Sakakura T., Yoshida T. Expression of tenascin-C in stromal cells of the murine uterus during early pregnancy: induction by interleukin-1 alpha, prostaglandin E(2), and prostaglandin F(2 alpha). Biol Reprod. 2000 Dec;63(6):1713–1720. doi: 10.1095/biolreprod63.6.1713. [DOI] [PubMed] [Google Scholar]
- Pfander D., Cramer T., Deuerling D., Weseloh G., Swoboda B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann Rheum Dis. 2000 Jun;59(6):448–454. doi: 10.1136/ard.59.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander D., Rahmanzadeh R., Scheller E. E. Presence and distribution of collagen II, collagen I, fibronectin, and tenascin in rabbit normal and osteoarthritic cartilage. J Rheumatol. 1999 Feb;26(2):386–394. [PubMed] [Google Scholar]
- Pfander D., Swoboda B., Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol. 2001 Nov;159(5):1777–1783. doi: 10.1016/S0002-9440(10)63024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander David, Cramer Thorsten, Schipani Ernestina, Johnson Randall S. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003 May 1;116(Pt 9):1819–1826. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]
- Pullig O., Weseloh G., Gauer S., Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol. 2000 Jul;19(3):245–255. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Pullig O., Weseloh G., Ronneberger D., Käkönen S., Swoboda B. Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif Tissue Int. 2000 Sep;67(3):230–240. doi: 10.1007/s002230001108. [DOI] [PubMed] [Google Scholar]
- Salter D. M. Tenascin is increased in cartilage and synovium from arthritic knees. Br J Rheumatol. 1993 Sep;32(9):780–786. doi: 10.1093/rheumatology/32.9.780. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Triantafillou S., Parker A., Youssef P. P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997 Feb;24(2):365–371. [PubMed] [Google Scholar]
- Smith R. L., Lin J., Trindade M. C., Shida J., Kajiyama G., Vu T., Hoffman A. R., van der Meulen M. C., Goodman S. B., Schurman D. J. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000 Mar-Apr;37(2):153–161. [PubMed] [Google Scholar]
- Stöve J., Huch K., Günther K. P., Scharf H. P. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000 May-Jun;68(3):144–149. doi: 10.1159/000055915. [DOI] [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Vignon E., Balblanc J. C., Mathieu P., Louisot P., Richard M. Metalloprotease activity, phospholipase A2 activity and cytokine concentration in osteoarthritis synovial fluids. Osteoarthritis Cartilage. 1993 Apr;1(2):115–120. doi: 10.1016/s1063-4584(05)80026-3. [DOI] [PubMed] [Google Scholar]
- Weller A., Beck S., Ekblom P. Amino acid sequence of mouse tenascin and differential expression of two tenascin isoforms during embryogenesis. J Cell Biol. 1991 Jan;112(2):355–362. doi: 10.1083/jcb.112.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.