Abstract
Objective: To investigate the effect of a single intravenous treatment with glucocorticoids (GC) encapsulated in long-circulating PEG-liposomes on both joint inflammation and cartilage destruction and to investigate the phenomenon of selective homing of these liposomes in the inflamed synovium.
Methods: Mice with collagen type II-induced arthritis (CIA) were intravenously treated with liposomal and free prednisolone phosphate (PLP) a few days after the first signs of the disease. Joint inflammation was scored during 1 week after treatment, after which sections of the knee joints were prepared for assessment of cartilage damage. In addition, arthritic mice were treated with liposomes containing colloidal gold. 24 hours after injection, knee joint sections were prepared in which the location of liposomes was visualised.
Results: Treatment of CIA with 10 mg/kg liposomal PLP resulted in a strong and lasting resolution of joint inflammation. 10 mg/kg free PLP only became slightly effective after repeated daily injections. Although joint inflammation recurred 1 week after treatment with liposomal PLP, knee joint sections prepared at this time indicated that the cartilage damage was still reduced. Localisation of gold labelled liposomes in the inflamed joints was seen in the proximity of blood vessels, in the cellular infiltrate, but mainly in the synovial lining. Unaffected joints did not take up liposomes.
Conclusions: By using the property of long-circulating liposomes to target the synovial lining selectively in inflamed joints, the anti-inflammatory activity of GC can be greatly increased, showing also the beneficial effect of reduced cartilage destruction.
Full Text
The Full Text of this article is available as a PDF (779.6 KB).
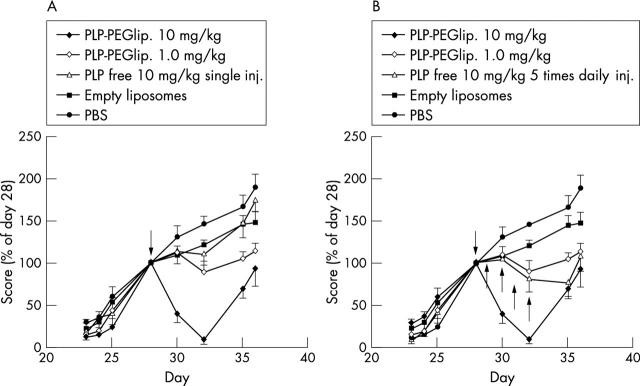
Figure 1 .
Paw inflammation scores after a single treatment with 10 mg/kg PLP-PEG-liposomes, 1 mg/kg PLP-PEG-liposomes, 10 mg/kg unencapsulated PLP, compared with both empty PEG-liposomes and PBS as controls. In contrast with liposomal PLP, a single treatment with 10 mg/kg unencapsulated PLP had no significant effect (A). Multiple treatment with five daily injections of 10 mg/kg unencapsulated PLP had a significant effect at days 32 and 35 but the effect was no better than that of 1 mg/kg PLP-PEG-liposomes (B). Each point represents the mean of 7 mice (SEM). Arrows indicate treatment.
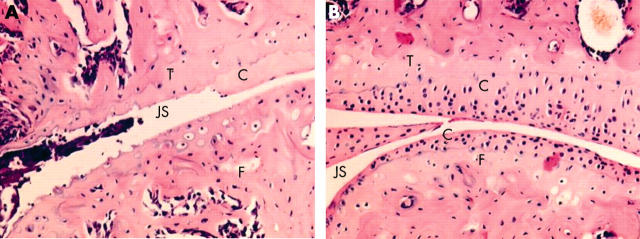
Figure 2 .
Effect of liposomal PLP on cartilage loss 1 week after treatment. (A) Knee joint section after treatment with saline; (B) the same knee joint after treatment with 10 mg/kg PLP-PEG-liposomes. Original magnification x200. Haematoxylin and eosin staining. T, tibia; F, femur, JS, joint space; C, cartilage layer.
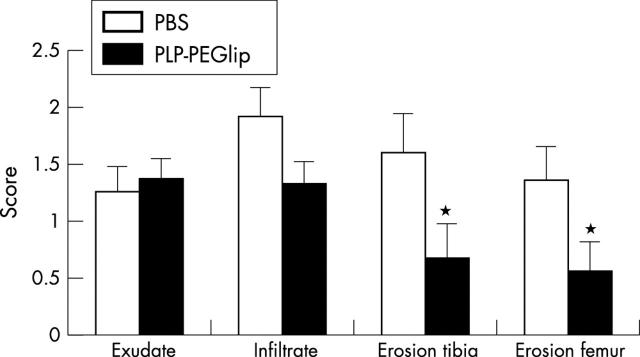
Figure 3 .
Histological evaluation of the effect on exudate and infiltrate as measures for inflammation, and on cartilage erosion, of 10 mg/kg liposomal PLP at 1 week after treatment. Data indicate the mean of seven mice (SEM). Asterisks indicate significance (p<0.05).
Figure 4 .
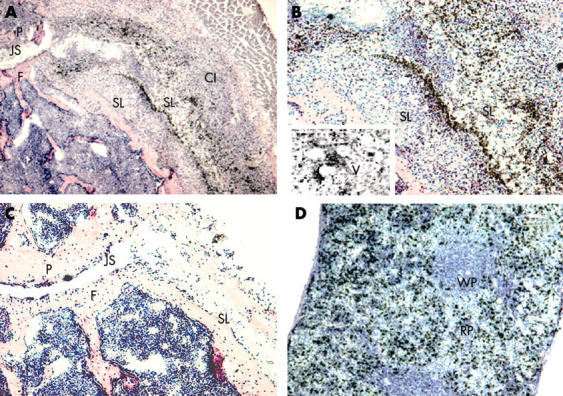
Visualisation of gold labelled PEG-liposomes. (A) Gold-liposomes in the inflamed knee joint. Original magnification x50. (B) Magnification x100 of an area surrounding the synovial lining. Insert: magnification x200 of an area surrounding blood vessels. Gold particles are visible as black dots. Note that the liposomal gold is mainly localised in the synovial lining and some around blood vessels. Relatively little gold is visible in the cellular infiltrate. (C) Visualisation of gold labelled PEG-liposomes in an unaffected knee joint. Original magnification x50. (D) Spleen localisation of gold labelled PEG liposomes. Original magnification x100. P, patella; F, femur, JS, joint space; CI, cellular infiltrate; SL, synovial lining; V, vessels; WP, white pulp; RP, red pulp.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahsan Fakhrul, Rivas Isabel P., Khan Mansoor A., Torres Suarez Ana I. Targeting to macrophages: role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J Control Release. 2002 Feb 19;79(1-3):29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- Amano Y., Lee S. W., Allison A. C. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: mediation by decreased mRNA stability. Mol Pharmacol. 1993 Feb;43(2):176–182. [PubMed] [Google Scholar]
- Barrera P., Blom A., van Lent P. L., van Bloois L., Beijnen J. H., van Rooijen N., de Waal Malefijt M. C., van de Putte L. B., Storm G., van den Berg W. B. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000 Sep;43(9):1951–1959. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Camilleri J. P., Williams A. S., Amos N., Douglas-Jones A. G., Love W. G., Williams B. D. The effect of free and liposome-encapsulated clodronate on the hepatic mononuclear phagocyte system in the rat. Clin Exp Immunol. 1995 Feb;99(2):269–275. doi: 10.1111/j.1365-2249.1995.tb05544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Sulli A., Barone A., Seriolo B., Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993 May-Jun;11(3):331–339. [PubMed] [Google Scholar]
- Hansen T. M., Dickmeiss E., Jans H., Ingemann Hansen T., Ingeman-Nielsen M., Lorenzen I. Combination of methylprednisolone pulse therapy and remission inducing drugs in rheumatoid arthritis. Ann Rheum Dis. 1987 Apr;46(4):290–295. doi: 10.1136/ard.46.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Huang S. K., Hong K., Lee K. D., Papahadjopoulos D., Friend D. S. Light microscopic localization of silver-enhanced liposome-entrapped colloidal gold in mouse tissues. Biochim Biophys Acta. 1991 Oct 14;1069(1):117–121. doi: 10.1016/0005-2736(91)90111-k. [DOI] [PubMed] [Google Scholar]
- Joyce D. A., Gimblett G., Steer J. H. Targets of glucocorticoid action on TNF-alpha release by macrophages. Inflamm Res. 2001 Jul;50(7):337–340. doi: 10.1007/PL00012387. [DOI] [PubMed] [Google Scholar]
- Kinne R. W., Bräuer R., Stuhlmüller B., Palombo-Kinne E., Burmester G. R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000 Apr 12;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverman P., Dams E. T., Storm G., Hafmans T. G., Croes H. J., Oyen W. J., Corstens F. H., Boerman O. C. Microscopic localization of PEG-liposomes in a rat model of focal infection. J Control Release. 2001 Aug 10;75(3):347–355. doi: 10.1016/s0168-3659(01)00402-3. [DOI] [PubMed] [Google Scholar]
- Laverman P, Boerman OC, Oyen WJ, Dams ET, Storm G, Corstens FH. Liposomes for scintigraphic detection of infection and inflammation. Adv Drug Deliv Rev. 1999 Apr 5;37(1-3):225–235. doi: 10.1016/s0169-409x(98)00095-7. [DOI] [PubMed] [Google Scholar]
- Liebling M. R., Leib E., McLaughlin K., Blocka K., Furst D. E., Nyman K., Paulus H. E. Pulse methylprednisolone in rheumatoid arthritis: a double-blind cross-over trial. Ann Intern Med. 1981 Jan;94(1):21–26. doi: 10.7326/0003-4819-94-1-21. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Chrousos G. P., Bornstein S. R. Enigmas of adrenal androgen and glucocorticoid dissociation in premenopausal onset rheumatoid arthritis. J Rheumatol. 1999 Feb;26(2):247–250. [PubMed] [Google Scholar]
- Metselaar Josbert M., Wauben Marca H. M., Wagenaar-Hilbers Josee P. A., Boerman Otto C., Storm Gert. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 2003 Jul;48(7):2059–2066. doi: 10.1002/art.11140. [DOI] [PubMed] [Google Scholar]
- Oku N., Namba Y. Long-circulating liposomes. Crit Rev Ther Drug Carrier Syst. 1994;11(4):231–270. [PubMed] [Google Scholar]
- Richards P. J., Williams A. S., Goodfellow R. M., Williams B. D. Liposomal clodronate eliminates synovial macrophages, reduces inflammation and ameliorates joint destruction in antigen-induced arthritis. Rheumatology (Oxford) 1999 Sep;38(9):818–825. doi: 10.1093/rheumatology/38.9.818. [DOI] [PubMed] [Google Scholar]
- Richards P. J., Williams B. D., Williams A. S. Suppression of chronic streptococcal cell wall-induced arthritis in Lewis rats by liposomal clodronate. Rheumatology (Oxford) 2001 Sep;40(9):978–987. doi: 10.1093/rheumatology/40.9.978. [DOI] [PubMed] [Google Scholar]
- Roujeau J. C. Pulse glucocorticoid therapy. The 'big shot' revisited. Arch Dermatol. 1996 Dec;132(12):1499–1502. [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Saag Kenneth G. Glucocorticoid use in rheumatoid arthritis. Curr Rheumatol Rep. 2002 Jun;4(3):218–225. doi: 10.1007/s11926-002-0068-z. [DOI] [PubMed] [Google Scholar]
- Schiffelers R. M., Storm G., Bakker-Woudenberg I. A. Host factors influencing the preferential localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue. Pharm Res. 2001 Jun;18(6):780–787. doi: 10.1023/a:1011080211226. [DOI] [PubMed] [Google Scholar]
- Snell E. S. The pharmacological properties of corticosteroids in relation to clinical efficacy. Br J Dermatol. 1976 Mar;94 Suppl 12:15–23. doi: 10.1111/j.1365-2133.1976.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Van Lent P. L., Holthuysen A. E., Van Rooijen N., Van De Putte L. B., Van Den Berg W. B. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998 Jul;57(7):408–413. doi: 10.1136/ard.57.7.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994 Sep 14;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Weusten B. L., Jacobs J. W., Bijlsma J. W. Corticosteroid pulse therapy in active rheumatoid arthritis. Semin Arthritis Rheum. 1993 Dec;23(3):183–192. doi: 10.1016/s0049-0172(05)80039-3. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Cuello C., Bertouch J. V., Roberts-Thomson P. J., Ahern M. J., Smith M. D., Youssef P. P. Effects of pulse methylprednisolone on macrophage chemotactic protein-1 and macrophage inflammatory protein-1alpha in rheumatoid synovium. J Rheumatol. 2001 Dec;28(12):2634–2636. [PubMed] [Google Scholar]
- Woodle M. C., Lasic D. D. Sterically stabilized liposomes. Biochim Biophys Acta. 1992 Aug 14;1113(2):171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- Woodle M. C., Newman M. S., Cohen J. A. Sterically stabilized liposomes: physical and biological properties. J Drug Target. 1994;2(5):397–403. doi: 10.3109/10611869408996815. [DOI] [PubMed] [Google Scholar]
- van Lent P. L., Holthuysen A. E., van den Bersselaar L., van Rooijen N., van de Putte L. B., van den Berg W. B. Role of macrophage-like synovial lining cells in localization and expression of experimental arthritis. Scand J Rheumatol Suppl. 1995;101:83–89. doi: 10.3109/03009749509100906. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van Lent P. L. The role of macrophages in chronic arthritis. Immunobiology. 1996 Oct;195(4-5):614–623. doi: 10.1016/S0171-2985(96)80026-X. [DOI] [PubMed] [Google Scholar]