Abstract
Background: Osteoclasts, specialised bone resorbing cells regulated by RANKL and M-CSF, are implicated in rheumatoid joint erosion. Lymphocyte-monocyte interactions activate bone resorption, this being attributed to tumour necrosis factor α (TNFα) and interleukin 1 ß (IL1ß) enhanced osteoblast expression of RANKL. In animal studies, TNF potently increases osteoclast formation in the presence of RANKL. RANKL-independent osteoclastogenesis also occurs, though IL1 is required for resorptive function in most studies. These inflammatory cytokines have a pivotal role in rheumatoid arthritis,
Objective: To study the interactions of TNFα and IL1ß with RANKL, particularly the time course of the interactions and the role of lymphocytes.
Method: Cultures of lymphocytes and monocytes (osteoclast precursors) or of purified CD14+ cells alone (osteoclast precursors) were exposed to various combinations of TNFα, RANKL, and IL1ß or the inhibitors osteoprotegerin, IL1 receptor antagonist, or neutralising antibodies to RANKL or to IL1. Osteoclastogenesis and resorptive activity were assessed on microscopy of dentine slices.
Results: TNFα potently increased osteoclast proliferation/differentiation in the presence of RANKL. This effect was greatest when RANKL was present before but not after exposure of osteoclast precursor cells to TNFα. The resorptive activity of osteoclasts generated by TNFα in the absence of RANKL was critically dependent upon IL1, which was expressed by lymphocyte-monocyte interaction.
Conclusion: TNFα potently enhances RANKL mediated osteoclast activity. Interactions between TNFα and IL1 also result in osteoclastic activity independently of RANKL. These findings will inform therapeutic approaches to the prevention of joint erosion in rheumatoid arthritis.
Full Text
The Full Text of this article is available as a PDF (499.8 KB).
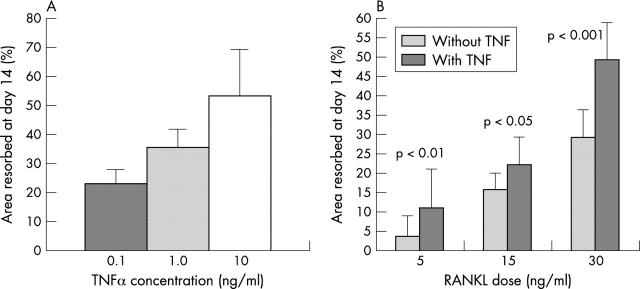
Figure 1 .
TNFα is synergistic with RANKL in osteoclast regulation. (A) Dose-resorption effect on area of osteoclastic resorption of dentine slices on adding TNFα in a range of doses to PBMCs cultured in RANKL 30 ng/ml and M-CSF 25 ng/ml. (B) Effect on area of osteoclastic resorption of dentine slices of adding TNFα 1 ng/ml to RANKL at 5, 15, or 30 ng/ml. Mean of four to six slices in three experiments; error bars are the standard deviation.
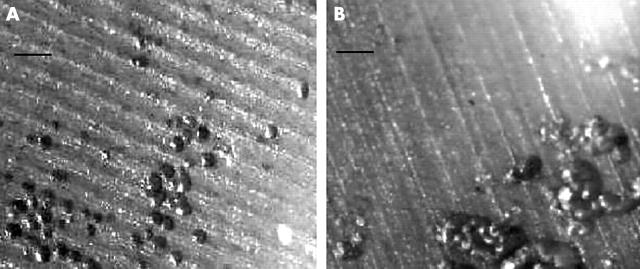
Figure 2 .
RANKL-independent osteoclast formation in cultures of PBMCs. Reflected light microscopy of dentine slices stained with toluidine blue showing individual and grouped resorption pits. (A) cells cultured in M-CSF and TNFα (1 ng/ml); (B) cells cultured in M-CSF, TNFα (1 ng/ml), and OPG (200 ng/ml). Bar = 50 µm.
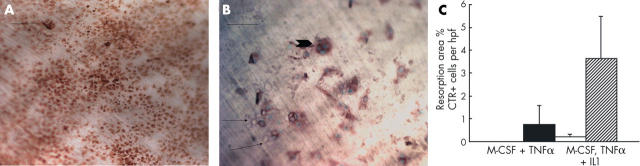
Figure 3 .
Osteoclastogenesis from CD14+ selected cells cultured in (A) M-CSF and TNFα or (B) in M-CSF, TNFα, and IL1. Addition of IL1 resulted in resorptive activity (pits shown by arrows) by CTR+ cells (arrow head), whereas in the absence of IL1, the CTR+ cells did not resorb. Bar (photomicrographs) = 50 µm. Staining by indirect immunoperoxidase method. Data from repeated experiments are shown in the graph (C). The number of CTR+ cells per high power field in the absence (black bar) or presence (hatched bar) of IL1 did not differ significantly. The resorption area was only measurable when IL1 was added (white bar). Error bars are the standard deviation.
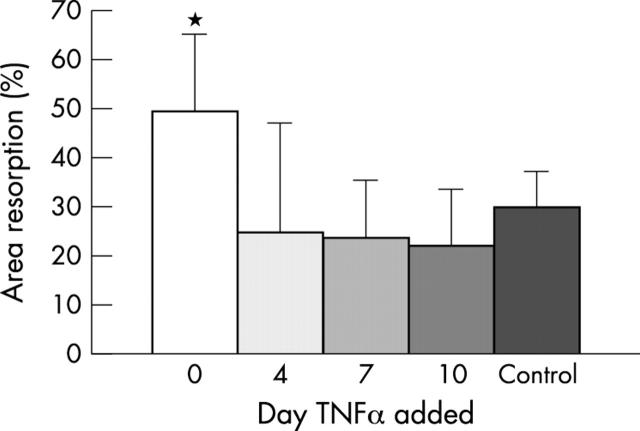
Figure 4 .
Effect of TNFα depends on timing of addition to cultures. PBMCs were cultured in M-CSF and RANKL, with TNF added at various time points. Significantly more resorption occurred when TNFα was added on day 0 (white bar) compared with control to which no TNFα was added (black bar), p<0.001. The resorption area was not increased when TNFα was added on day 4, 7 or 10. Mean of six slices in three experiments; error bars are the standard deviation.
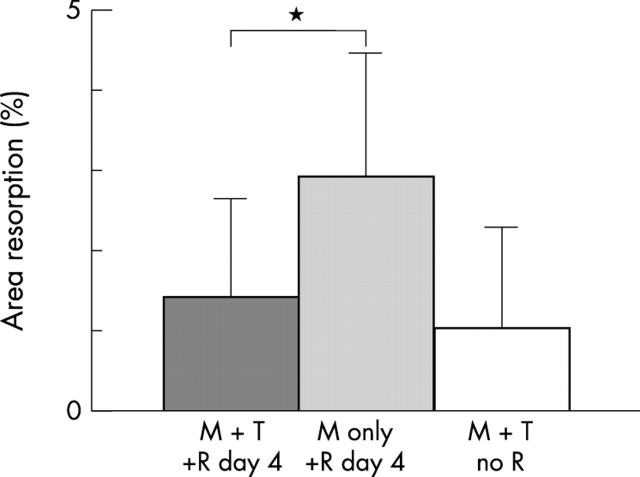
Figure 5 .
Exposure to TNFα before RANKL reduces osteoclastic resorption activity. PBMCs were cultured in M-CSF (light grey bar) or in M-CSF with TNFα (dark grey bar) before adding RANKL after 4 days, and compared with cells treated with M-CSF and TNFα alone (white bar). Osteoclast resorption was reduced when cells were exposed to TNFα before RANKL (*p = 0.08). Mean of six slices in three experiments; error bars are the standard deviation. M, M-CSF 25 ng/ml; T, TNFα 1 ng/ml; R, RANKL 30 ng/ml.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma Y., Kaji K., Katogi R., Takeshita S., Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000 Feb 18;275(7):4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986 Feb 6;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Durand J. M., Buchs N., Fossiez F., Page G., Frappart L., Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999 May;42(5):963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Chabaud M., Miossec P. The combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 2001 Jun;44(6):1293–1303. doi: 10.1002/1529-0131(200106)44:6<1293::AID-ART221>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Crotti T. N., Smith M. D., Weedon H., Ahern M. J., Findlay D. M., Kraan M., Tak P. P., Haynes D. R. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002 Dec;61(12):1047–1054. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer Jean-Michel, Bresnihan Barry. Targeting interleukin-1 in the treatment of rheumatoid arthritis. Arthritis Rheum. 2002 Mar;46(3):574–578. doi: 10.1002/art.10168. [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Stashenko P. P., Mole J. E., Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985 Oct;135(4):2562–2568. [PubMed] [Google Scholar]
- Erwig L. P., Kluth D. C., Walsh G. M., Rees A. J. Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol. 1998 Aug 15;161(4):1983–1988. [PubMed] [Google Scholar]
- Fox S. W., Fuller K., Bayley K. E., Lean J. M., Chambers T. J. TGF-beta 1 and IFN-gamma direct macrophage activation by TNF-alpha to osteoclastic or cytocidal phenotype. J Immunol. 2000 Nov 1;165(9):4957–4963. doi: 10.4049/jimmunol.165.9.4957. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y., Sabokbar A., Neale S. D., Itonaga I., Torisu T., Athanasou N. A. The effect of macrophage-colony stimulating factor and other humoral factors (interleukin-1, -3, -6, and -11, tumor necrosis factor-alpha, and granulocyte macrophage-colony stimulating factor) on human osteoclast formation from circulating cells. Bone. 2001 Mar;28(3):261–267. doi: 10.1016/s8756-3282(00)00453-1. [DOI] [PubMed] [Google Scholar]
- Fuller Karen, Murphy Chiho, Kirstein Barrie, Fox Simon W., Chambers Timothy J. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002 Mar;143(3):1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Galson D. L., Goldring S. R., Auron P. E. The role of TNF-receptor family members and other TRAF-dependent receptors in bone resorption. Arthritis Res. 2000 Nov 2;3(1):6–12. doi: 10.1186/ar134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Harada Y., Wang J. T., Gorn A. H., Thornhill T. S., Goldring S. R. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998 Apr;152(4):943–951. [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Manning C., Tsay A., Naito A., Pan C., Amento E., Goldring S. R. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000 Feb;43(2):250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Danks L., Sabokbar A., Athanasou N. A. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford) 2002 Nov;41(11):1232–1239. doi: 10.1093/rheumatology/41.11.1232. [DOI] [PubMed] [Google Scholar]
- Hofbauer L. C., Lacey D. L., Dunstan C. R., Spelsberg T. C., Riggs B. L., Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999 Sep;25(3):255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Mergenhagen S. E., Raisz L. G. Macrophage-lymphocyte synergy in the production of osteoclast activating factor. J Immunol. 1974 Oct;113(4):1278–1287. [PubMed] [Google Scholar]
- Kobayashi K., Takahashi N., Jimi E., Udagawa N., Takami M., Kotake S., Nakagawa N., Kinosaki M., Yamaguchi K., Shima N. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000 Jan 17;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine M., Kukita A., Kukita T., Ogata Y., Hotokebuchi T., Kohashi O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001 May;28(5):474–483. doi: 10.1016/s8756-3282(01)00420-3. [DOI] [PubMed] [Google Scholar]
- Kong Y. Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S., Capparelli C., Li J., Elliott R., McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 Nov 18;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Kudo Osami, Fujikawa Yosuke, Itonaga Ichiro, Sabokbar Afsie, Torisu Takehiko, Athanasou Nicholas A. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol. 2002 Oct;198(2):220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- Lader C. S., Flanagan A. M. Prostaglandin E2, interleukin 1alpha, and tumor necrosis factor-alpha increase human osteoclast formation and bone resorption in vitro. Endocrinology. 1998 Jul;139(7):3157–3164. doi: 10.1210/endo.139.7.6085. [DOI] [PubMed] [Google Scholar]
- Lam J., Takeshita S., Barker J. E., Kanagawa O., Ross F. P., Teitelbaum S. L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000 Dec;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., van der Heijde D. M., St Clair E. W., Furst D. E., Breedveld F. C., Kalden J. R., Smolen J. S., Weisman M., Emery P., Feldmann M. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000 Nov 30;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Massey H. M., Scopes J., Horton M. A., Flanagan A. M. Transforming growth factor-beta1 (TGF-beta) stimulates the osteoclast-forming potential of peripheral blood hematopoietic precursors in a lymphocyte-rich microenvironment. Bone. 2001 Jun;28(6):577–582. doi: 10.1016/s8756-3282(01)00432-x. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., Malakellis M., Collier F. M., Cameron P. U., Holloway W. R., Gough T. J., Gregorio-King C., Kirkland M. A., Myers D. E. Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL). Clin Sci (Lond) 2000 Aug;99(2):133–140. [PubMed] [Google Scholar]
- Ogata Y., Kukita A., Kukita T., Komine M., Miyahara A., Miyazaki S., Kohashi O. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J Immunol. 1999 Mar 1;162(5):2754–2760. [PubMed] [Google Scholar]
- Pettit A. R., Ji H., von Stechow D., Müller R., Goldring S. R., Choi Y., Benoist C., Gravallese E. M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001 Nov;159(5):1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas E., Bakharevski O., Hards D. K., Kartsogiannis V., Quinn J. M., Ryan P. F., Martin T. J., Gillespie M. T. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000 Apr;43(4):821–826. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sabokbar A., Kudo O., Athanasou N. A. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J Orthop Res. 2003 Jan;21(1):73–80. doi: 10.1016/S0736-0266(02)00106-7. [DOI] [PubMed] [Google Scholar]
- Shigeyama Y., Pap T., Kunzler P., Simmen B. R., Gay R. E., Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000 Nov;43(11):2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000 Dec;43(12):2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum D. E. T cells: pathogenic cells and therapeutic targets in rheumatoid arthritis. Semin Arthritis Rheum. 1999 Aug;29(1):27–35. doi: 10.1016/s0049-0172(99)80035-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Heulsmann A., Tondravi M. M., Mukherjee A., Abu-Amer Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001 Jan 5;276(1):563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]