Abstract
Objective: To examine the in vivo effects of carboxymethylated chitin (CMC), intra-articularly administered, on cartilage degradation and the level and distribution of cartilage matrix metalloproteinase-1 (MMP-1).
Methods: Osteoarthritis (OA) was induced in 20 rabbits by unilateral anterior cruciate ligament transection (ACLT). The experimental group, comprising 10 rabbits randomly selected, was given an intra-articular injection of 0.3 ml of 2% CMC solution at 1, 3, and 5 weeks after ACLT. A further 10 rabbits that received an intra-articular injection of 0.3 ml normal saline at the same time served as controls. All knees were harvested at 6 weeks after surgery. Cartilage degradation of femoral condyles was evaluated at two levels: macroscopic and light microscopic. Tissue level and distribution of MMP-1 was documented by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry.
Results: Cartilage degradation in the control group was significantly more severe than that in the experimental group both on the macroscopic grading scale and on Mankin's grading scale. In RT-PCR the amount of MMP-1 was significantly reduced by the treatment of CMC. Immunohistochemical study showed that in the experimental group MMP-1 was predominantly expressed in the superficial and upper intermediate layers of cartilage, and the amount of MMP-1 in the experimental group was also lower than that in control group.
Conclusion: CMC significantly reduces the severity of cartilage degradation and reduces the expression of MMP-1 in cartilage, both at the mRNA and the protein level, and thus may be a potential drug for the treatment of OA.
Full Text
The Full Text of this article is available as a PDF (281.8 KB).
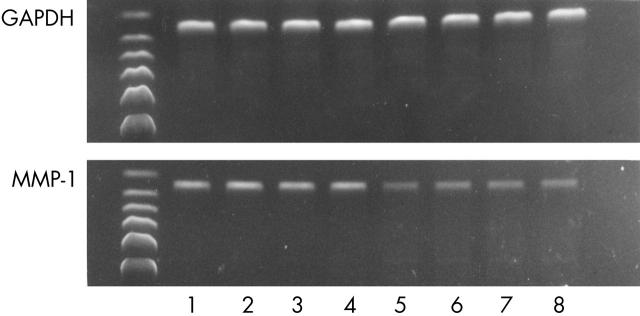
Figure 1 .
Representative bands of MMP-1 in RT-PCR electrophoresis (lanes 1–4, control group; lanes 5–8, experimental group): indicating that the expression of MMP-1 in the experimental group is significantly lower than that in control group. GAPDH is a reference gene; the lengths of the MMP-1 and GAPDH bands are 307 bp and 302 bp, respectively.
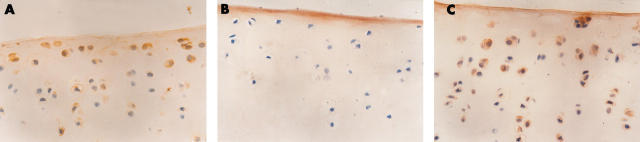
Figure 2 .
Immunohistochemistry study of MMP-1 (photomicrograph x400). (A) The experimental group, showing that MMP-1 positive cells are predominantly located in the superficial and upper intermediate layers. (B) The immunohistochemical control, showing only background staining. (C) The control group, showing that the number of MMP-1 positive cells is significantly increased compared with that in the experimental group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt K. D., Braunstein E. M., Visco D. M., O'Connor B., Heck D., Albrecht M. Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol. 1991 Mar;18(3):436–446. [PubMed] [Google Scholar]
- Deal C. L., Moskowitz R. W. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate. Rheum Dis Clin North Am. 1999 May;25(2):379–395. doi: 10.1016/s0889-857x(05)70074-0. [DOI] [PubMed] [Google Scholar]
- Han F., Ishiguro N., Ito T., Sakai T., Iwata H. Effects of sodium hyaluronate on experimental osteoarthritis in rabbit knee joints. Nagoya J Med Sci. 1999 Nov;62(3-4):115–126. [PubMed] [Google Scholar]
- Hashimoto S., Takahashi K., Amiel D., Coutts R. D., Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998 Jul;41(7):1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hollander A. P., Pidoux I., Reiner A., Rorabeck C., Bourne R., Poole A. R. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995 Dec;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner J. L., Otterness I. G., Freund E. M., Caterson B., Kraus V. B. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998 May;41(5):877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Hwang S. M., Chen C. Y., Chen S. S., Chen J. C. Chitinous materials inhibit nitric oxide production by activated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2000 Apr 29;271(1):229–233. doi: 10.1006/bbrc.2000.2602. [DOI] [PubMed] [Google Scholar]
- Ishiguro N., Ito T., Ito H., Iwata H., Jugessur H., Ionescu M., Poole A. R. Relationship of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover: analyses of synovial fluid from patients with osteoarthritis. Arthritis Rheum. 1999 Jan;42(1):129–136. doi: 10.1002/1529-0131(199901)42:1<129::AID-ANR16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jin G., Sah R. L., Li Y. S., Lotz M., Shyy J. Y., Chien S. Biomechanical regulation of matrix metalloproteinase-9 in cultured chondrocytes. J Orthop Res. 2000 Nov;18(6):899–908. doi: 10.1002/jor.1100180608. [DOI] [PubMed] [Google Scholar]
- Klein R., Becker E. W., Berg P. A., Bernau A. Immunomodulatory properties of rumalon, a glycosaminoglycan peptide complex, in patients with osteoarthritis: activation of T helper cell type 2 cytokines and antigen-specific IgG4 antigen-specific igG4 antibodies. J Rheumatol. 2000 Feb;27(2):448–454. [PubMed] [Google Scholar]
- Lahiji A., Sohrabi A., Hungerford D. S., Frondoza C. G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000 Sep 15;51(4):586–595. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lu J. X., Prudhommeaux F., Meunier A., Sedel L., Guillemin G. Effects of chitosan on rat knee cartilages. Biomaterials. 1999 Oct;20(20):1937–1944. doi: 10.1016/s0142-9612(99)00097-6. [DOI] [PubMed] [Google Scholar]
- Malemud C. J., Goldberg V. M. Future directions for research and treatment of osteoarthritis. Front Biosci. 1999 Oct 15;4:D762–D771. doi: 10.2741/malemud. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Pelletier J. P., Faure M. P., DiBattista J. A., Wilhelm S., Visco D., Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Jovanovic D., Fernandes J. C., Manning P., Connor J. R., Currie M. G., Di Battista J. A., Martel-Pelletier J. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998 Jul;41(7):1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Lascau-Coman V., Jovanovic D., Fernandes J. C., Manning P., Connor J. R., Currie M. G., Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J Rheumatol. 1999 Sep;26(9):2002–2014. [PubMed] [Google Scholar]
- Sechriest V. F., Miao Y. J., Niyibizi C., Westerhausen-Larson A., Matthew H. W., Evans C. H., Fu F. H., Suh J. K. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000 Mar 15;49(4):534–541. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Suh J. K., Matthew H. W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000 Dec;21(24):2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Hashimoto S., Kubo T., Hirasawa Y., Lotz M., Amiel D. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000 Jul;27(7):1713–1720. [PubMed] [Google Scholar]
- Tetlow L. C., Adlam D. J., Woolley D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001 Mar;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]