Abstract
Background: Anti-filaggrin antibodies (AFA) are among the most specific antibodies for rheumatoid arthritis, so procedures for their detection should be included in early biological diagnoses. AFA can be detected by indirect immunofluorescence (anti-keratin antibodies, AKA) or by new enzyme immunoassays (EIA). Their comparative performance needs to be established.
Objective: To compare these technical procedures to optimise the serological diagnosis of rheumatoid arthritis.
Methods: Results obtained using AKA and EIA were compared in 271 sera from 140 patients with rheumatoid arthritis at various stages, 98 patients with other autoimmune diseases, and 33 healthy subjects. EIA were successively undertaken with citrullinated linear filaggrin peptide (home made EIA) or cyclic citrullinated peptide (CCP2, commercial kits). Rheumatoid factor (RF) was assessed by EIA in all patients.
Results: Anti-CCP2 kits showed the best sensitivity and specificity (65% and 96%, respectively). Among the 140 patients with rheumatoid arthritis, those with very recent disease (less than six months' duration, n = 21) were studied as a separate group. In this group, the sensitivity of anti-CCP2 kits decreased to ~50%. Nevertheless this assay remained the most accurate when compared with AKA or home made EIA using linear filaggrin peptides. The combination of anti-CCP2 and RF only slightly increased the sensitivity of the diagnosis of very early rheumatoid arthritis.
Conclusions: Kits using citrullinated cyclic peptides (CCP2) were more suitable than either AKA or EIA using linear filaggrin peptides for the diagnosis of early rheumatoid disease.
Full Text
The Full Text of this article is available as a PDF (273.3 KB).
Figure 1 .
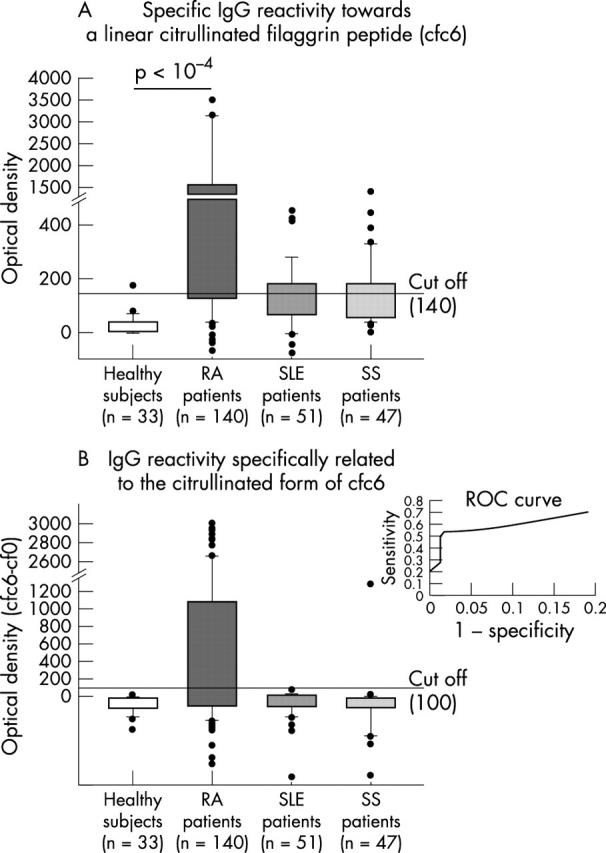
(A) Analysis of the serum IgG response to the citrullinated filaggrin peptide (cfc6) in different populations of subjects. Patients with rheumatoid arthritis had a significantly higher serum IgG response to cfc6 than those with other connective diseases or the healthy controls. However, a cut off value established at OD 140 (mean optical density value of the healthy subjects +3 SD) did not accurately discriminate the patients with rheumatoid arthritis from those with the other connective tissue diseases. IgG anti-filaggrin peptide was detected by this procedure in more than 30% of the cases of Sjogren's syndrome and SLE. Results are expressed as mean optical densities in the reactive well subtracted from the OD in blank well. (B) Evaluation of the serum IgG response related to the citrullinated form of cfc6 in different populations. Subtraction of the optical density of the IgG response to cf0 from that of the IgG reactivity to cfc6 discriminated the IgG response of the rheumatoid arthritis group to filaggrin peptide. A receiver operating characteristic (ROC) curve (insert) established a cut off value at 100 arbitrary units that discriminated rheumatoid arthritis patients with a sensitivity of 50% and a specificity of 98%. The boundaries of the boxes indicate the 25th and 75th centiles; whiskers above and below the boxes indicate the 90th and 10th centiles. RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Wells G., Verhoeven A. C., Felson D. T. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000 Jan;43(1):22–29. doi: 10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bas S., Perneger T. V., Mikhnevitch E., Seitz M., Tiercy J. M., Roux-Lombard P., Guerne P. A. Association of rheumatoid factors and anti-filaggrin antibodies with severity of erosions in rheumatoid arthritis. Rheumatology (Oxford) 2000 Oct;39(10):1082–1088. doi: 10.1093/rheumatology/39.10.1082. [DOI] [PubMed] [Google Scholar]
- Bas S., Perneger T. V., Seitz M., Tiercy J-M, Roux-Lombard P., Guerne P. A. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 2002 Jul;41(7):809–814. doi: 10.1093/rheumatology/41.7.809. [DOI] [PubMed] [Google Scholar]
- Berthelot J. M., Maugars Y., Prost A., Youinou P. Rheumatoid arthritis and filaggrin. A review. Rev Rhum Engl Ed. 1995 Feb;62(2):127–138. [PubMed] [Google Scholar]
- Bizzaro N., Mazzanti G., Tonutti E., Villalta D., Tozzoli R. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. Clin Chem. 2001 Jun;47(6):1089–1093. [PubMed] [Google Scholar]
- Buckland-Wright J. C., Clarke G. S., Chikanza I. C., Grahame R. Quantitative microfocal radiography detects changes in erosion area in patients with early rheumatoid arthritis treated with myocrisine. J Rheumatol. 1993 Feb;20(2):243–247. [PubMed] [Google Scholar]
- Cervera R., Font J., Ramos-Casals M., García-Carrasco M., Rosas J., Morlà R. M., Muñoz F. J., Artigues A., Pallarés L., Ingelmo M. Primary Sjögren's syndrome in men: clinical and immunological characteristics. Lupus. 2000;9(1):61–64. doi: 10.1177/096120330000900111. [DOI] [PubMed] [Google Scholar]
- Cordonnier C., Meyer O., Palazzo E., de Bandt M., Elias A., Nicaise P., Haïm T., Kahn M. F., Chatellier G. Diagnostic value of anti-RA33 antibody, antikeratin antibody, antiperinuclear factor and antinuclear antibody in early rheumatoid arthritis: comparison with rheumatoid factor. Br J Rheumatol. 1996 Jul;35(7):620–624. doi: 10.1093/rheumatology/35.7.620. [DOI] [PubMed] [Google Scholar]
- Egsmose C., Lund B., Borg G., Pettersson H., Berg E., Brodin U., Trang L. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol. 1995 Dec;22(12):2208–2213. [PubMed] [Google Scholar]
- Ferraro-Peyret Carole, Tebib Jacques, Desbos Agnes, Bihannic Rene, Genestier Christelle, Letroublon Marie-Claude, Veber Sophie, Benoit Etienne, Veysseyre-Balter Cecile, Monier Jean-Claude. Improvement in diagnosis of rheumatoid arthritis using dual indirect immunofluorescence and immunoblotting assays for antifilaggrin autoantibodies: a retrospective 3 year study. J Rheumatol. 2002 Feb;29(2):276–281. [PubMed] [Google Scholar]
- Girbal-Neuhauser E., Durieux J. J., Arnaud M., Dalbon P., Sebbag M., Vincent C., Simon M., Senshu T., Masson-Bessière C., Jolivet-Reynaud C. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999 Jan 1;162(1):585–594. [PubMed] [Google Scholar]
- Goldbach-Mansky R., Lee J., McCoy A., Hoxworth J., Yarboro C., Smolen J. S., Steiner G., Rosen A., Zhang C., Ménard H. A. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000 Mar 31;2(3):236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroot E. J., de Jong B. A., van Leeuwen M. A., Swinkels H., van den Hoogen F. H., van't Hof M., van de Putte L. B., van Rijswijk M. H., van Venrooij W. J., van Riel P. L. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000 Aug;43(8):1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Masson-Bessière C., Sebbag M., Girbal-Neuhauser E., Nogueira L., Vincent C., Senshu T., Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001 Mar 15;166(6):4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- Meyer O., Labarre C., Dougados M., Goupille Ph, Cantagrel A., Dubois A., Nicaise-Roland P., Sibilia J., Combe B. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003 Feb;62(2):120–126. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro R., Hampson R., McEntegart A., Thomson E. A., Madhok R., Capell H. Improved functional outcome in patients with early rheumatoid arthritis treated with intramuscular gold: results of a five year prospective study. Ann Rheum Dis. 1998 Feb;57(2):88–93. doi: 10.1136/ard.57.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell James R. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002 Feb;46(2):283–285. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- Palosuo T., Tilvis R., Strandberg T., Aho K. Filaggrin related antibodies among the aged. Ann Rheum Dis. 2003 Mar;62(3):261–263. doi: 10.1136/ard.62.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraux Alain, Berthelot Jean M., Chalès Gérard, Le Henaff Catherine, Mary Jean Y., Thorel Jean B., Hoang Sylvie, Dueymes Maryvonne, Allain Jérôme, Devauchelle Valerie. Value of laboratory tests in early prediction of rheumatoid arthritis. Arthritis Rheum. 2002 Apr 15;47(2):155–165. doi: 10.1002/art.10241. [DOI] [PubMed] [Google Scholar]
- Schellekens G. A., Visser H., de Jong B. A., van den Hoogen F. H., Hazes J. M., Breedveld F. C., van Venrooij W. J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000 Jan;43(1):155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schellekens G. A., de Jong B. A., van den Hoogen F. H., van de Putte L. B., van Venrooij W. J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998 Jan 1;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Girbal E., Sebbag M., Gomès-Daudrix V., Vincent C., Salama G., Serre G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called "antikeratin antibodies," autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993 Sep;92(3):1387–1393. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack S. L., Mannik M., Dale B. A. Diagnostic value of antibodies to filaggrin in rheumatoid arthritis. J Rheumatol. 1998 May;25(5):847–851. [PubMed] [Google Scholar]
- Symmons D. P., Jones M. A., Scott D. L., Prior P. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol. 1998 Jun;25(6):1072–1077. [PubMed] [Google Scholar]
- Union Ann, Meheus Lydie, Humbel René Louis, Conrad Karsten, Steiner Guenter, Moereels Henri, Pottel Hans, Serre Guy, De Keyser Filip. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002 May;46(5):1185–1195. doi: 10.1002/art.10229. [DOI] [PubMed] [Google Scholar]
- Vasiliauskiene L., Wiik A., Høier-Madsen M. Prevalence and clinical significance of antikeratin antibodies and other serological markers in Lithuanian patients with rheumatoid arthritis. Ann Rheum Dis. 2001 May;60(5):459–466. doi: 10.1136/ard.60.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasishta Anil. Diagnosing early-onset rheumatoid arthritis: the role of anti-CCP antibodies. Am Clin Lab. 2002 Aug-Sep;21(7):34–36. [PubMed] [Google Scholar]
- Vincent C., de Keyser F., Masson-Bessière C., Sebbag M., Veys E. M., Serre G. Anti-perinuclear factor compared with the so called "antikeratin" antibodies and antibodies to human epidermis filaggrin, in the diagnosis of arthritides. Ann Rheum Dis. 1999 Jan;58(1):42–48. doi: 10.1136/ard.58.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser Henk, le Cessie Saskia, Vos Koen, Breedveld Ferdinand C., Hazes Johanna M. W. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002 Feb;46(2):357–365. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F. A comparison of IgM rheumatoid factor by nephelometry and latex methods: clinical and laboratory significance. Arthritis Care Res. 1998 Apr;11(2):89–93. doi: 10.1002/art.1790110204. [DOI] [PubMed] [Google Scholar]
- van der Heide A., Jacobs J. W., Bijlsma J. W., Heurkens A. H., van Booma-Frankfort C., van der Veen M. J., Haanen H. C., Hofman D. M., van Albada-Kuipers G. A., ter Borg E. J. The effectiveness of early treatment with "second-line" antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996 Apr 15;124(8):699–707. doi: 10.7326/0003-4819-124-8-199604150-00001. [DOI] [PubMed] [Google Scholar]


