Abstract
Background: CD4+ T lymphocytes play an important part in the pathogenesis of scleroderma (systemic sclerosis, SSc) and predominate in perivascular SSc skin lesions. Both soluble and membrane bound adhesion molecules are overexpressed in SSc, possibly influencing lymphocyte/endothelial cell (EC) contact.
Objective: To assess the transendothelial migration capacity of peripheral lymphocytes in vitro.
Patients and methods: Collagen was covered with human umbilical vein endothelial cells (HUVEC), and peripheral blood mononuclear cells (PBMC) of patients and matched healthy controls (HC) were added in parallel experiments. Before and after fractionated harvest of non-adherent, bound, and migrated lymphocytes, the CD4/CD8 ratio and the lymphocytic expression of activation markers and adhesion molecules were analysed by fluorocytometry.
Results: 13 (SD 12)% of the SSc PBMC migrated compared with only 5 (5)% HC PBMC (p<0.0002); this increase was primarily due to the migration of CD3+ T lymphocytes and mainly to a larger proportion of CD4+ cells within this CD3+ fraction (71 (SD 14)% for SSc v 56 (14)% for HC, p<0.03), leading to an increased CD4/CD8 ratio among migrated SSc lymphocytes in comparison with controls (3.3 (1.5) v 1.62 (0.93), p<0.006). Among migrated SSc CD4+ T lymphocytes, the frequency of HLA-DR+ cells was increased; migrated lymphocytes highly expressed the adhesion molecules CD11a, CD49d, CD29, and CD44.
Conclusion: Transendothelial migration of CD4+ T lymphocytes is enhanced in SSc, and migrating cells exhibit an activated phenotype. The data suggest that activated CD3+CD4+ lymphocytes as found in SSc peripheral blood are prone to transvascular migration, thus contributing to the formation of typical perivascular lymphocytic infiltrates.
Full Text
The Full Text of this article is available as a PDF (269.3 KB).
Figure 1 .
Assay of transendothelial migration. HUVEC after the third to fourth passage formed confluent monolayers on collagen gels after overnight incubation. On the second day of the experiment, we added fresh PBMC, obtained from SSc and matched HC and processed them in parallel experiments. After 1 hour of migration we analysed the harvested cell fractions (NAD, BND, MIG) and ex vivo stained PBMC by fluorocytometry. NAD, non-adherent; BND, bound; MIG, migrated, EC, endothelial cells, PBMC peripheral blood mononuclear cells.
Figure 2 .
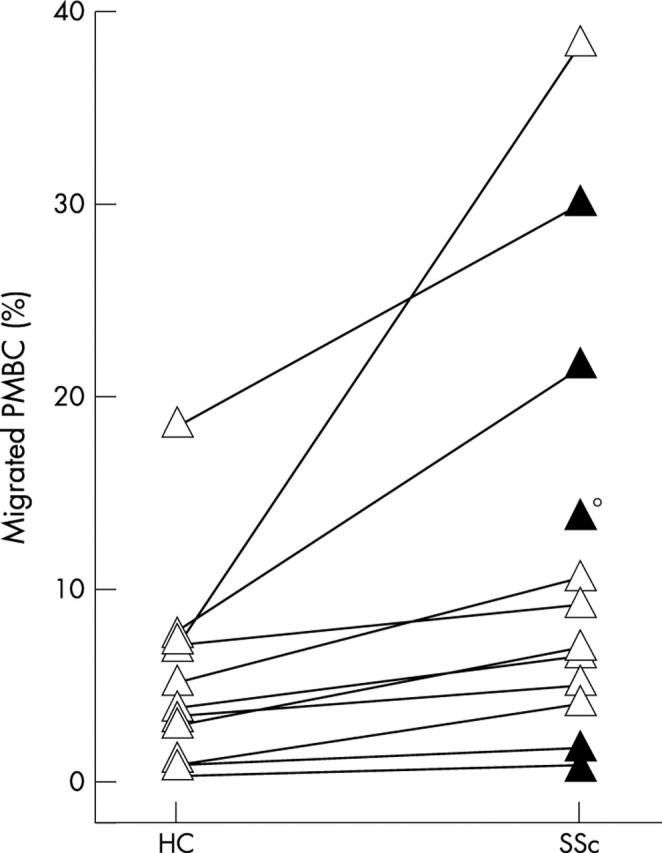
Increased transendothelial migration of SSc PBMC. Testing PBMC of SSc and HC in parallel experiments. Filled triangles indicate diffuse SSc, open triangles indicate limited disease, and the small circle indicates that the respective negative control had highly activated lymphocytes and developed influenza the next day. This single sample was therefore excluded from all analyses.
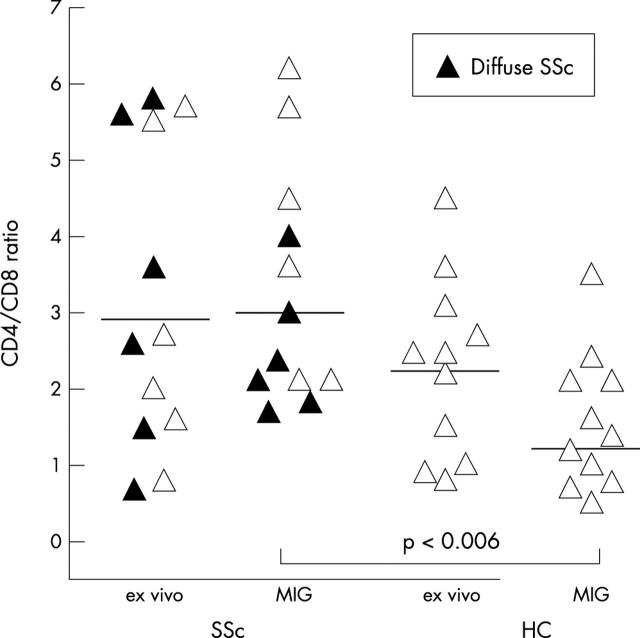
Figure 3 .
Rise in the CD4/CD8 ratio in migrated SSc lymphocytes. The CD4/CD8 ratio in patients with SSc was only slightly raised ex vivo (p = NS), but increased in 8/12 patients with SSc and decreased in 10/11 HC, leading to a significantly raised CD4/CD8 ratio in migrated lymphocytes of patients with SSc when compared with HC (for details see table 2).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aringer M., Wintersberger W., Steiner C. W., Kiener H., Presterl E., Jaeger U., Smolen J. S., Graninger W. B. High levels of bcl-2 protein in circulating T lymphocytes, but not B lymphocytes, of patients with systemic lupus erythematosus. Arthritis Rheum. 1994 Oct;37(10):1423–1430. doi: 10.1002/art.1780371004. [DOI] [PubMed] [Google Scholar]
- Brezinschek R. I., Oppenheimer-Marks N., Lipsky P. E. Activated T cells acquire endothelial cell surface determinants during transendothelial migration. J Immunol. 1999 Feb 1;162(3):1677–1684. [PubMed] [Google Scholar]
- Claman H. N., Giorno R. C., Seibold J. R. Endothelial and fibroblastic activation in scleroderma. The myth of the "uninvolved skin". Arthritis Rheum. 1991 Dec;34(12):1495–1501. doi: 10.1002/art.1780341204. [DOI] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Pietschmann P., Oppenheimer-Marks N., Lipsky P. E. The intrinsic migratory capacity of memory T cells contributes to their accumulation in rheumatoid synovium. Arthritis Rheum. 1992 Dec;35(12):1434–1444. doi: 10.1002/art.1780351206. [DOI] [PubMed] [Google Scholar]
- Degiannis D., Seibold J. R., Czarnecki M., Raskova J., Raska K., Jr Soluble and cellular markers of immune activation in patients with systemic sclerosis. Clin Immunol Immunopathol. 1990 Aug;56(2):259–270. doi: 10.1016/0090-1229(90)90147-i. [DOI] [PubMed] [Google Scholar]
- Denton C. P., Shi-Wen X., Sutton A., Abraham D. J., Black C. M., Pearson J. D. Scleroderma fibroblasts promote migration of mononuclear leucocytes across endothelial cell monolayers. Clin Exp Immunol. 1998 Nov;114(2):293–300. doi: 10.1046/j.1365-2249.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagała-Kulawik J., Hoser G., Kawalec M., Doboszyńska A., Kawiak J., Droszcz W. Lymphocyte phenotyping in systemic sclerosis: a flow cytometry analysis of lymphocytes in bronchoalveolar lavage fluid. Anal Quant Cytol Histol. 1997 Jun;19(3):264–270. [PubMed] [Google Scholar]
- Eghtesad M., Jackson H. E., Cunningham A. C. Primary human alveolar epithelial cells can elicit the transendothelial migration of CD14+ monocytes and CD3+ lymphocytes. Immunology. 2001 Feb;102(2):157–164. doi: 10.1046/j.1365-2567.2001.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst D. E., Clements P. J. Hypothesis for the pathogenesis of systemic sclerosis. J Rheumatol Suppl. 1997 May;48:53–57. [PubMed] [Google Scholar]
- Furst D. E. Rational therapy in the treatment of systemic sclerosis. Curr Opin Rheumatol. 2000 Nov;12(6):540–544. doi: 10.1097/00002281-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Galley H. F., Blaylock M. G., Dubbels A. M., Webster N. R. Variability in E-selectin expression, mRNA levels and sE-selectin release between endothelial cell lines and primary endothelial cells. Cell Biol Int. 2000;24(2):91–99. doi: 10.1006/cbir.1999.0455. [DOI] [PubMed] [Google Scholar]
- Galéa P., Brezinschek R., Lipsky P. E., Oppenheimer-Marks N. Phenotypic characterization of CD4-/alpha beta TCR+ and gamma delta TCR+ T cells with a transendothelial migratory capacity. J Immunol. 1994 Jul 15;153(2):529–542. [PubMed] [Google Scholar]
- Gorla R., Airò P., Malagoli A., Carella G., Prati E., Brugnoni D., Franceschini F., Cattaneo R. CD4+ and CD8+ subsets: naive and memory cells in the peripheral blood of patients with systemic sclerosis. Clin Rheumatol. 1994 Mar;13(1):83–87. doi: 10.1007/BF02229871. [DOI] [PubMed] [Google Scholar]
- Grisar J., Hahn P., Brosch S., Peterlik M., Smolen J. S., Pietschmann P. Phenotypic characteristics of human monocytes undergoing transendothelial migration. Arthritis Res. 2001 Jan 11;3(2):127–132. doi: 10.1186/ar150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruschwitz M., von den Driesch P., Kellner I., Hornstein O. P., Sterry W. Expression of adhesion proteins involved in cell-cell and cell-matrix interactions in the skin of patients with progressive systemic sclerosis. J Am Acad Dermatol. 1992 Aug;27(2 Pt 1):169–177. doi: 10.1016/0190-9622(92)70165-c. [DOI] [PubMed] [Google Scholar]
- Gustafsson R., Tötterman T. H., Klareskog L., Hällgren R. Increase in activated T cells and reduction in suppressor inducer T cells in systemic sclerosis. Ann Rheum Dis. 1990 Jan;49(1):40–45. doi: 10.1136/ard.49.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahaleh M. B., Fan P. S. Mechanism of serum-mediated endothelial injury in scleroderma: identification of a granular enzyme in scleroderma skin and sera. Clin Immunol Immunopathol. 1997 Apr;83(1):32–40. doi: 10.1006/clin.1996.4322. [DOI] [PubMed] [Google Scholar]
- Kahaleh M. B., Sultany G. L., Smith E. A., Huffstutter J. E., Loadholt C. B., LeRoy E. C. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986 Oct-Dec;4(4):367–369. [PubMed] [Google Scholar]
- Klein C. L., Bittinger F., Köhler H., Wagner M., Otto M., Hermanns I., Kirkpatrick C. J. Comparative studies on vascular endothelium in vitro. 3. Effects of cytokines on the expression of E-selectin, ICAM-1 and VCAM-1 by cultured human endothelial cells obtained from different passages. Pathobiology. 1995;63(2):83–92. doi: 10.1159/000163938. [DOI] [PubMed] [Google Scholar]
- Klein C. L., Köhler H., Bittinger F., Wagner M., Hermanns I., Grant K., Lewis J. C., Kirkpatrick C. J. Comparative studies on vascular endothelium in vitro. I. Cytokine effects on the expression of adhesion molecules by human umbilical vein, saphenous vein and femoral artery endothelial cells. Pathobiology. 1994;62(4):199–208. doi: 10.1159/000163911. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M. Activation of dermal connective tissue in scleroderma. Ann Med. 1993 Dec;25(6):511–518. [PubMed] [Google Scholar]
- Kähäri V. M., Sandberg M., Kalimo H., Vuorio T., Vuorio E. Identification of fibroblasts responsible for increased collagen production in localized scleroderma by in situ hybridization. J Invest Dermatol. 1988 May;90(5):664–670. doi: 10.1111/1523-1747.ep12560826. [DOI] [PubMed] [Google Scholar]
- Lidington E. A., Moyes D. L., McCormack A. M., Rose M. L. A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl Immunol. 1999 Dec;7(4):239–246. doi: 10.1016/s0966-3274(99)80008-2. [DOI] [PubMed] [Google Scholar]
- Majewski S., Hunzelmann N., Johnson J. P., Jung C., Mauch C., Ziegler-Heitbrock H. W., Riethmüller G., Krieg T. Expression of intercellular adhesion molecule-1 (ICAM-1) in the skin of patients with systemic scleroderma. J Invest Dermatol. 1991 Oct;97(4):667–671. doi: 10.1111/1523-1747.ep12483739. [DOI] [PubMed] [Google Scholar]
- Meheus L., van Venrooij W. J., Wiik A., Charles P. J., Tzioufas A. G., Meyer O., Steiner G., Gianola D., Bombardieri S., Union A. Multicenter validation of recombinant, natural and synthetic antigens used in a single multiparameter assay for the detection of specific anti-nuclear autoantibodies in connective tissue disorders. Clin Exp Rheumatol. 1999 Mar-Apr;17(2):205–214. [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Pietschmann P., Cush J. J., Lipsky P. E., Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992 Aug 15;149(4):1170–1178. [PubMed] [Google Scholar]
- Prescott R. J., Freemont A. J., Jones C. J., Hoyland J., Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992 Mar;166(3):255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- Raab Markus, Daxecker Heide, Markovic Snezana, Karimi Alireza, Griesmacher Andrea, Mueller Mathias M. Variation of adhesion molecule expression on human umbilical vein endothelial cells upon multiple cytokine application. Clin Chim Acta. 2002 Jul;321(1-2):11–16. doi: 10.1016/s0009-8981(02)00048-7. [DOI] [PubMed] [Google Scholar]
- Roumm A. D., Whiteside T. L., Medsger T. A., Jr, Rodnan G. P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984 Jun;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- Sgonc R., Gruschwitz M. S., Boeck G., Sepp N., Gruber J., Wick G. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000 Nov;43(11):2550–2562. doi: 10.1002/1529-0131(200011)43:11<2550::AID-ANR24>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sollberg S., Peltonen J., Uitto J., Jimenez S. A. Elevated expression of beta 1 and beta 2 integrins, intercellular adhesion molecule 1, and endothelial leukocyte adhesion molecule 1 in the skin of patients with systemic sclerosis of recent onset. Arthritis Rheum. 1992 Mar;35(3):290–298. doi: 10.1002/art.1780350307. [DOI] [PubMed] [Google Scholar]
- Steen V. D., Medsger T. A., Jr Epidemiology and natural history of systemic sclerosis. Rheum Dis Clin North Am. 1990 Feb;16(1):1–10. [PubMed] [Google Scholar]
- Stummvoll G. H., Aringer M., Smolen J. S., Köller M., Kiener H. P., Steiner C. W., Bohle B., Knobler R., Graninger W. B. Derangement of apoptosis-related lymphocyte homeostasis in systemic sclerosis. Rheumatology (Oxford) 2000 Dec;39(12):1341–1350. doi: 10.1093/rheumatology/39.12.1341. [DOI] [PubMed] [Google Scholar]
- Valentini G., Della Rossa A., Bombardieri S., Bencivelli W., Silman A. J., D'Angelo S., Cerinic M. M., Belch J. F., Black C. M., Bruhlmann P. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis. 2001 Jun;60(6):592–598. doi: 10.1136/ard.60.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A. U., Lorimer S., Majumdar S., Harrison N. K., Corrin B., Black C. M., Jeffery P. K., du Bois R. M. Fibrosing alveolitis in systemic sclerosis: increase in memory T-cells in lung interstitium. Eur Respir J. 1995 Feb;8(2):266–271. doi: 10.1183/09031936.95.08020266. [DOI] [PubMed] [Google Scholar]
- White B. Immunologic aspects of scleroderma. Curr Opin Rheumatol. 1995 Nov;7(6):541–545. doi: 10.1097/00002281-199511000-00013. [DOI] [PubMed] [Google Scholar]
- White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996 Nov;22(4):695–708. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- White B., Yurovsky V. V. Oligoclonal expansion of V delta 1+ gamma/delta T-cells in systemic sclerosis patients. Ann N Y Acad Sci. 1995 Jul 7;756:382–391. doi: 10.1111/j.1749-6632.1995.tb44542.x. [DOI] [PubMed] [Google Scholar]
- Yurovsky V. V. The repertoire of T-cell receptors in systemic sclerosis. Crit Rev Immunol. 1995;15(2):155–165. doi: 10.1615/critrevimmunol.v15.i2.30. [DOI] [PubMed] [Google Scholar]