Abstract
By using both genetic and biochemical approaches, we have investigated the physiological role of Shp-2, a cytoplasmic tyrosine phosphatase with two Src homology 2 domains, in signaling pathways downstream of epidermal growth factor receptor (EGF-R). In previous studies, a targeted deletion mutation in the SH2-N domain of Shp-2 was introduced into the murine Shp-2 locus, which resulted in embryonic lethality of homozygous mutant (Shp-2−/−) mice at midgestation. By aggregating Shp-2−/− embryonic stem cells with wild-type embryos, we created Shp-2−/−/wild-type chimeric animals. Most chimeras had open eyelids at birth and abnormal skin development, a phenotype characteristic of mice with mutations in EGF-R signaling components. In genetic crosses, a heterozygous Shp-2 mutation dominantly enhanced the phenotype of a weak mutant allele of EGF-R (wa-2), resulting in distinctive growth retardation, developmental defects in the skin, lung, and intestine, and perinatal mortality that are reminiscent of EGF-R knockout mice. Biochemical analysis revealed that signal propagation proximal to the EGF-R upon EGF stimulation was significantly attenuated in wa-2 fibroblast cells, which was exacerbated by the additional Shp-2 mutation. Thus, we provide biological evidence here that protein-tyrosine phosphatase Shp-2 acts to enhance information flow from the EGF-R in mouse growth and development.
Shp-2 is a widely expressed cytoplasmic phospho-tyrosine phosphatase that contains two Src homology 2 (SH2) domains at the N terminus. Despite a wealth of biochemical data suggesting that Shp-2 participates in signal transduction through a number of receptor tyrosine kinases (1, 2), specific involvement of Shp-2 in a growth factor-initiated signaling pathway in mammalian system has not been well addressed. Genetic analysis of the Shp-2 homologue, Corkscrew (Csw), in Drosophila has indicated that Csw acts downstream of Torso in embryonic body organization and formation of terminal structures and also in the Sevenless pathway for the control of R7 photoreceptor cell differentiation (3–6). Microinjection of mutant Shp-2 mRNA molecules into Xenopus embryos revealed a putative role of Shp-2 in fibroblast growth factor-induced mesodermal induction, presumably through the extracellular signal-regulated kinase (Erk) pathway (7). However, the physiological function of Shp-2 in mammals remains largely unknown. We demonstrated previously that deletion of 65 aa in the N-terminal SH2 (SH2-N) domain of Shp-2 gave rise to a loss-of-function mutation that resulted in embryonic lethality of homozygous mutant (Shp-2−/−) animals around day 8.5–10.5 (8). Mutant embryos failed to gastrulate properly, with a range of severe developmental defects in node, notochord, and posterior structures.
The early embryonic lethality of mutant mice, which appears to be a common phenotype of mutations in many signaling proteins that are widely expressed, made it difficult to define the Shp-2 involvement in specific signaling pathways. To address this issue, we isolated homozygous mutant (Shp-2−/−) embryonic stem (ES) cells for in vitro ES cell differentiation assay and chimeric animal analysis (9–11). Shp-2−/− ES cells displayed a dramatically decreased ability to differentiate into mature erythroid and myeloid cell lineages in vitro (9). Consistently, there was almost no contribution of Shp-2−/− cells to hematopoietic progenitor cells arising in Shp-2−/−/wild-type (WT) chimeric animals (11). These results strongly suggest that Shp-2 is an indispensable positive regulator in blood cell development, which is in contrast to the negative effect of Shp-1 in hematopoiesis (12–15). In addition, we have observed that the Shp-2 mutation leads to abnormal limb development, consistent with the notion that Shp-2 participates in signaling from fibroblast growth factor receptor (7, 11).
In this report, we present biological evidence that Shp-2 is a critical signal-enhancing factor downstream of the epidermal growth factor receptor (EGF-R). Many Shp-2−/−/WT chimeras displayed a distinct phenotype of defective EGF-R signaling, open eyelids at birth and abnormal skin architecture. Furthermore, the subtle phenotype of a weak mutant allele of EGF-R (waved-2, wa-2) was dominantly enhanced by the targeted mutation at the Shp-2 locus, pointing to a specific requirement of Shp-2 for EGF-R-initiated signaling pathways.
MATERIALS AND METHODS
Generation of Chimeric Animals.
By using a gene targeting approach, we created a mutant Shp-2 allele with the deletion of exon 3, coding for amino acids 46–110 in the SH2-N domain of Shp-2, in murine ES cells (8). ES cells of 129/Sv origin, heterozygous (Shp-2+/−), or homozygous (Shp-2−/−) for the Shp-2 deletion mutation, were aggregated with WT CD1 embryos at the eight-cell stage to generate chimeric animals (11, 16). Chimeric pups were identified at birth by the agouti color of the eyes. Southern blot or PCR analysis of genomic DNA to detect the mutant allele also was performed to confirm the chimerism (9, 11).
Mouse Strains and Crossing.
Mutant mice with a targeted deletion of amino acids 46–110 in the SH2-N domain of Shp-2 were generated as described (8). Waved-2 (wa-2, B6EiC3H-a/A-Egfrwa-2vt) mice were obtained from the Jackson Laboratory. Mutant mice heterozygous for the Shp-2 mutation (Shp-2+/−) on the C57BL/6 background were mated with wa-2 mice (wa-2/wa-2). wa-2/+:Shp-2+/− mice were identified among F1 offspring. These mice were backcrossed with wa-2/wa-2 animals. F2 pups at birth and at weaning were genotyped and carefully examined. Newborns with premature eyelid opening were randomly sampled and separately genotyped. A distinctive curly whiskers and wavy coat phenotype was used to distinguish wa-2 homozygotes from heterozygotes. For PCR analysis, mouse tails or other tissues were lysed in a lysis buffer (100 mM Tris⋅HCl, pH 8.5/5 mM EDTA/0.2% SDS/200 mM NaCl/200 μg/ml proteinase K) for genomic DNA extraction. Genomic DNA (200 ng) was used as template for PCR amplification. After 35 cycles of amplification, the PCR products were examined by electrophoresis on 1.5% agarose gel. The primer E1A1 (GTA GGA GCC CTA TAG AAT CTG) and the primer PCR neoβ2 (TAC CCG GTA GAA TTG ACC TGC AG) were used to detect the Shp-2 mutant allele; another pair of primers (Shp-2–10: GAG TCA CAC AGA TCG TAT GCA TCC CA and Shp-2–11: GAT ACG CCT TCT CTC AAT GGA C) were designed to genotype the WT Shp-2 allele (8).
Histopathological Analysis.
Whole embryos or surgically removed tissue samples from animals were fixed in 10% buffered formalin, dehydrated through graded alcohol solutions, embedded in paraffin, sectioned at 5 μm, and processed for hematoxylin/eosin staining following standard protocols.
Derivation of Primary Fibroblast Cells and Biochemical Assays.
Newborn young mice from the intercross between wa-2/wa-2 and wa-2/+:Shp-2+/− were sacrificed. Subcutaneous tissues were surgically removed, minced, and digested by trypsin at 37°C for 20 min. Dissociated cells then were plated onto tissue culture plates with DMEM containing 15% FCS. Remaining cells or tissues were used for genotyping as described above. For the Erk kinase assay, cells were serum-starved for 24 hr and stimulated with EGF (100 ng/ml) for 5, 10, 20, or 60 min. Cell lysates were prepared and subjected to in vitro Erk kinase assay as reported (17). Immunoprecipitation and immunoblot analyses were done as described (17, 18). Rabbit polyclonal EGF-R antibody (1005) and anti-Cbl (C-15) antibody were purchased from Santa Cruz Biotechnology; anti-Shc antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Rabbit anti-SHPS-1 (Src Homology-containing phosphatase substrate-1) antibody was generously provided by Takashi Matozaki (Kobe University, Japan).
For phosphoinositide 3 (PI3) kinase assay, control and EGF-treated cells were lysed in RIPA buffer (0.15 M NaCl/0.05 M Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) (18), and cell lysates (750 μg) were precipitated with antibody against the p85 subunit of PI-3 kinase (Upstate Biotechnology). The assay was performed basically following the Upstate Biotechnology protocol. Briefly, immunoprecipitates were collected and washed twice with HNTG buffer (20 mM Hepes/150 mM NaCl/10% glycerol/0.1% Triton X-100) (17), twice with 100 mM Tris⋅HCl, pH 7.4/5 mM LiCl/0.1 mM sodium orthovanadate, and eventually twice with TNE buffer (10 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5 mM EDTA/0.1 mM sodium orthovanadate). The kinase reactions were carried out at 37°C for 10 min in 50 μl of TNE, 10 μl (20 μg/ml) of phosphatidylinositol, 10 μl of 100 mM MgCl2, and 5 μl of γ-32P-ATP working stock solution (0.88 mM ATP, 10 μci of γ-32P-ATP, 20 mM MgCl2), and were terminated by adding 20 μl of 6 N HCl. Radio-labeled lipid was extracted by adding 160 μl of CHCl3/MeOH (1:1), and the organic phase was separated from the aqueous phase by centrifugation. Samples (50 μl) from the lower organic phase were spotted onto 1% potassium oxalate-treated silicon gel 60 TLC plates (Merck) and resolved by chromatography in the solvent system of CHCl3/MeOH/H2O/NH4OH (60:47:11.3:2). Radio-labeled lipid was visualized by x-ray autophotography.
RESULTS
Distinct Eye and Skin Abnormality in Shp-2−/−/WT Chimeric Animals.
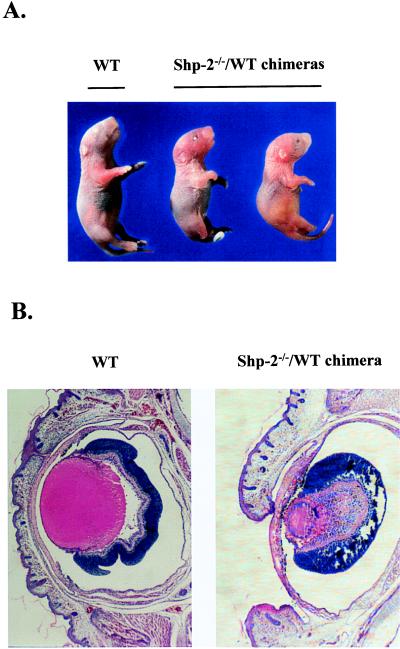
Isolation of Shp-2−/− ES cell lines and generation of Shp-2−/−/WT chimeric animals, using a morula aggregation technique, has been described in detail (9, 11). In Shp-2−/−/WT chimeras, one prominent abnormality was their distinctive eye defect, open eyelid, at birth (Fig. 1A). Because of multiple defects in a variety of organs in heavily chimeric animals (11), two-thirds of Shp-2−/−/WT chimeras were found dead at birth or died within 2 days after birth. Among the dead chimeras, 50% had open eyelids in one or both eyes caused by a delay in epithelial maturation (Table 1). A similar phenotype was seen in EGF-R and transforming growth factor type α knockout mice (19–23). This observation suggests that the deletion mutation in the Shp-2 gene leads to a specific defect in EGF-R signaling. In contrast, none of the Shp-2+/−/WT chimeric mice derived from Shp-2+/− ES cells and WT embryos died prematurely or had eye abnormalities (Table 1).
Figure 1.
Eye abnormality in newborn Shp-2−/−/WT chimeras. Chimeric mice were generated by aggregating Shp-2−/− ES cells with WT CD-1 embryos as described in the text. (A) One nonchimeric WT and two Shp-2−/−/WT chimeric newborns with open eyelid are shown. (B) Frontal section through an affected eye of a Shp-2−/−/WT chimeric newborn (Right) and a nonchimeric WT control (Left). The delayed fusion of eyelids was evident in the mutant eye. The lens was adherent to the cornea and iris, disorganized, and vacuolated. The cornea was thickened with the sign of neovascularization.
Table 1.
Abnormal eye and limb development in Shp-2−/−/WT chimeras
|
Shp-2−/−
|
Shp-2+/− | Shp-2+/+ | ||
|---|---|---|---|---|
| Dead | Alive | Alive | Alive | |
| Total number | 28 | 14 | 10 | 5 |
| of newborns | ||||
| Open eyelid | 14 | 4 | 0 | 0 |
| Hind limb | 22 | 8 | 0 | 0 |
| abnormality | ||||
Shp-2+/+, Shp-2+/− and Shp-2−/− ES cells were used to generate chimeric mice. Newborn pups were carefully examined under a dissecting microscope. Open eyelids were recorded for all newborns, either dead or alive. Hind leg abnormality includes limb truncation and incomplete digit formation.
Histological examination of affected “open” eyes from Shp-2−/−/WT animals revealed multiple defects, whereas “closed” eyes from nonchimeras appeared normal and well developed when sectioned (Fig. 1B). Compared with the control, an affected eye had a thickened cornea and fragments of pigmented tissue, with the sign of neovascularization. The lens was reduced in size, vacuolated, and filled with disorganized fibers, and it also was prolapsed into the anterior chamber and became adherent to the cornea and iris. Overall, the eye defects in the cornea, retina, and anterior chamber closely resembled those observed in transforming growth factor type α-deficient mice as well as in wa-2 mice that are also heterozygous for Sos1 mutation (21, 22, 24).
In addition to problems in eye structure, defective skin development was observed in Shp-2−/−/WT chimeras. Histological examination was undertaken on skins taken from the middorsal region of Shp-2−/−/WT and nonchimeric animals. Differences were apparent in skin sections of newborn pups, with disorganized and poorly developed hair follicles located in the thin dermal layer of Shp-2−/−/WT mice, similar to what was seen in wa-2/wa-2:Shp-2+/− animals (see Fig. 3B), as described below.
Figure 3.
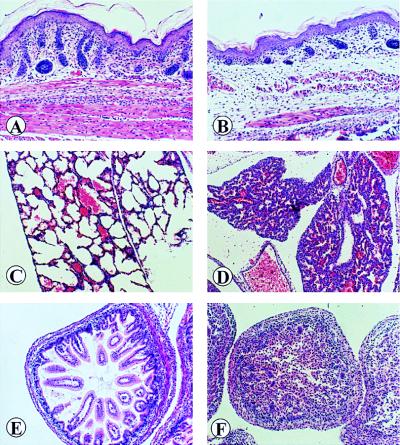
Defects in the skin, lung, and intestine of wa-2/Wa-2:Shp-2+/− mice. A comparative study was undertaken between newborn animals of wa-2/wa-2:Shp-2+/+ (A, C, and E) and wa-2/wa-2:Shp-2+/− origins (B, D, and F). (A and B) Section of abdomenal skins. In severely affected wa-2/wa-2:Shp-2+/− mouse, skin was poorly developed with no or few disorganized hair follicles, thinner epidermis, reduced subcutaneous fat or muscle tissue mass. Hematoxylin/eosin ×280. (C and D) Lung sections. There was a marked reduction in the size of alveolar spaces (atelectasis) and an increase in the thickness of alveolar walls in the lung of wa-2/wa-2:Shp-2+/− mice. Hematoxylin/eosin ×200. (E and F) Small bowel. The lumen is filled with desquamated cells, giving a solid appearance. The intestinal villi could not be visualized in the double mutant as compared with the control. Hematoxylin/eosin ×280.
Dominant Enhancement of a Weak EGF-R Allele by Heterozygous Shp-2 Mutation.
The results described above point to a positive regulatory role of Shp-2 in EGF-R-initiated intracellular signaling. To corroborate this observation, we conducted genetic analysis on the interaction between the mutant Shp-2 allele and a weak mutant allele of EGF-R (wa-2). wa-2 mice contain a single-nucleotide transversion resulting in a valine to glycine substitution at residue 743 in the kinase domain of the EGF-R (25, 26). Ligand-dependent autophosphorylation of wa-2 EGF-R is decreased by 5- to 10-fold, and the ability of wa-2 EGF-R to phosphorylate an exogenous substrate also is significantly decreased, compared with that of a WT EGF-R, even though the ligand-binding ability is not altered. wa-2 mice are well developed and fertile, with a subtle phenotype of wavy fur and curly whiskers. Perinatal lethality and open eyelids at birth occur only sporadically in wa-2 mice.
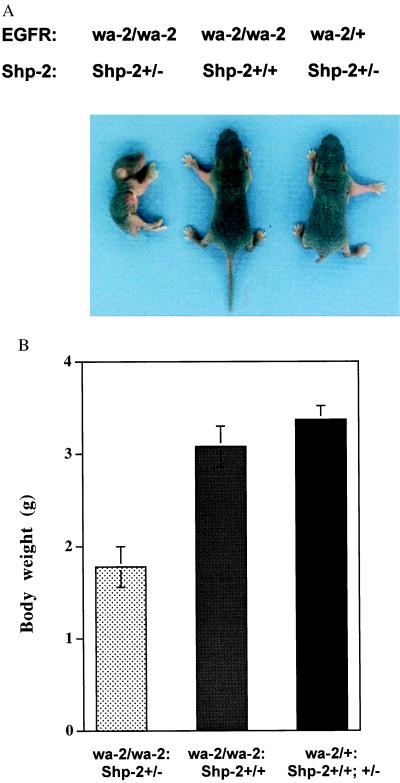
Mice heterozygous for the Shp-2 mutation (Shp-2+/−) appear developmentally normal and are indistinguishable from WT animals. Shp-2+/− mice were intercrossed with wa-2/wa-2 mice, and the resulting wa-2/+:Shp-2+/− mice were identified and backcrossed with wa-2/wa-2:Shp-2+/+ mice to generate offsprings with four possible genotypes. As shown in Table 2, genotyping at weaning showed a non-Mendelian distribution of offspring, because equal numbers of each genotype (wa-2/wa-2:Shp-2+/−, wa-2/wa-2:Shp-2+/+, wa-2/+:Shp-2+/−, and wa-2/+:Shp-2+/+) were expected from the crossing. Only 30% of wa-2/wa-2:Shp-2+/− animals survived to weaning, whereas the survival of wa-2/wa-2:Shp-2+/+ pups was about 82%. In subsequent experiments, we genotyped pups at birth and detected animals of four genotypes almost at expected Mendelian frequency (Table 2). This result indicates that wa-2 mutation induced a sporadic perinatal lethality, which is consistent with previous observations (24–27), and that the heterozygous Shp-2 mutation significantly enhanced the penetrance of this phenotype, although it did not lead to prenatal lethality. The wa-2 mice heterozygous for the Shp-2 mutation (wa-2/wa-2:Shp-2+/−) often died within 10 days after birth; they were malnourished and exhibited growth retardation and progressive wasting, as compared with their littermates of wa-2/wa-2:Shp-2+/+, wa-2/+:Shp-2+/−, and wa-2/+:Shp-2+/+ origins (Fig. 2 A and B). Furthermore, of 22 newborn F2 pups with the open-eyelid phenotype, the majority were wa-2/wa-2:Shp-2+/− mice, with only three of them genotyped as wa-2/wa-2:Shp-2+/+ (Table 2). This result suggests that the Shp-2 mutation increased the penetrance of the open-eyelid phenotype, which is sporadic in wa-2/wa-2 mice.
Table 2.
Heterozygous mutation in Shp-2 dominantly enhanced the phenotype of wa-2 mutation
| Wa-2/Wa-2:Shp-2+/− | Wa-2/Wa-2:Shp-2+/+ | Wa-2/+:Shp-2+/+ Wa-2/+:Shhp-2+/− | |
|---|---|---|---|
| Pups at weaning | 15 | 40 | 98 |
| Pups at birth | 32 | 35 | 71 |
| Pups with open | 19 | 3 | 0 |
| eyelid at birth |
wa-2/+:Shp-2+/−mice were crossed with wa-2/wa-2 mice to generate offsprings of four genotypes: wa-2/wa-2:Shp-2+/−, wa-2/wa-2:Shp-2+/+, wa-2/+:Shp-2+/−, and wa-2/+:Shp-2+/+ at an expected distribution of 1:1:1:1. In the first experiment, genotyping of mice was performed at weaning. In the second experiment, pups were genotyped at birth, and the frequency of open eyelids in newborns was scored simultaneously.
Figure 2.
Growth retardation of wa-2/wa-2:Shp-2+/− mice. wa-2/wa-2:Shp-2+/− mice were malnourished and displayed progressive wasting. (A) Representative animals of each genotype. (B) Comparison of the body weights of littermate mice at 4 days of age (n = 4). Around 70% of the wa-2/wa-2 mice with the Shp-2+/− mutation died 7–10 days after birth (see Table 2).
Multiple Defects Associated with Poor Epithelial Development in wa-2/wa-2:Shp-2+/− Mice.
We performed a detailed histological analysis on sections of skin, lung, and intestine for a comparison between wa-2/wa-2:Shp-2+/+ and wa-2/wa-2:Shp-2+/− mice (Fig. 3). There was a minor defect in the skin of wa-2/wa-2:Shp-2+/+ mice, as evidenced by some disoriented hair follicles that apparently account for the wavy fur in wa-2 mice (Fig. 3A). More severe defects were observed in the skin of wa-2/wa-2:Shp-2+/− mice, characterized by poorly developed and disordered hair follicles, a hypotrophy in the epidermis as well as a dramatic decrease in the size of subcutaneous muscle and fat tissues (Fig. 3B). Consistent with these architectural abnormalities, the thin skin of wa-2/wa-2:Shp-2+/− mice often appeared almost transparent and gradually became dry and flaky, with little hair outgrowth.
There were no obvious abnormalities in the lung and intestine of wa-2/wa-2:Shp-2+/+ mice (Fig. 3 C and E). However, consistent with their breathing difficulties, the lungs of dead wa-2/wa-2:Shp-2+/− mice were immaturely developed with poorly inflated areas. Sections of these mutant lungs also exhibited atelectasis with increased thickness and cell masses of the alveolar septae (Fig. 3D). In previous experiments, we had observed atelectatic lungs with hypercellularity of the alveolar septae in Shp-2−/−/WT chimeric animals (11). Similar pathological changes often are seen in immature human babies with respiratory distress syndrome (28). The lumen of stomach and small and large bowels of wa-2/wa-2:Shp-2+/− mice were filled with desquamated epithelial cells, suggesting lack of peristalsis (Fig. 3F). The muscle layer of the bowel was poorly organized and showed a significant reduction in thickness. The pathological disorders in the skin, lung, and intestine in wa-2/wa-2:Shp-2+/− mice were remarkably similar to that of EGF-R-deficient animals with a C57BL/6 genetic background (19, 20, 23). Together, these results suggest that heterozygous mutation of Shp-2 is a dominant enhancer of the wa-2 mutation toward the phenotype of EGF-R null mutant mice.
Shp-2 Is a Positive Player in Signaling Events Proximal to the EGF-R.
Ligand-activated EGF-R transduces signals by recruiting and phosphorylating a number of cytoplasmic proteins (29). Notably, the Shc proteins are believed to make a connection between EGF-R and the Ras-Erk pathway by association with the Grb2-Sos complex in a tyrosine phosphorylation-dependent fashion (30–32). The transmembrane protein SHPS-1 (SIRPα1) is phosphorylated and complexed with Shp-2 in response to growth factors such as EGF (33–35). Cbl, the product of c-cbl protooncogene, is another prominent target of EGF-R, and tyrosine-phosphorylated Cbl forms complexes with Shc, PI3 kinase, and Crk proteins (36, 37). Activation of PI3 kinase is crucial for generation of second messengers in EGF-R signaling (38).
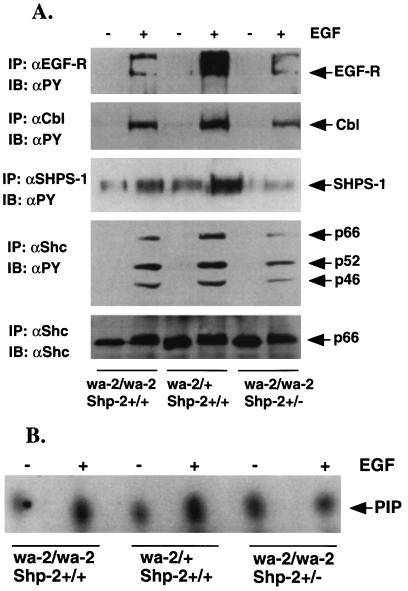
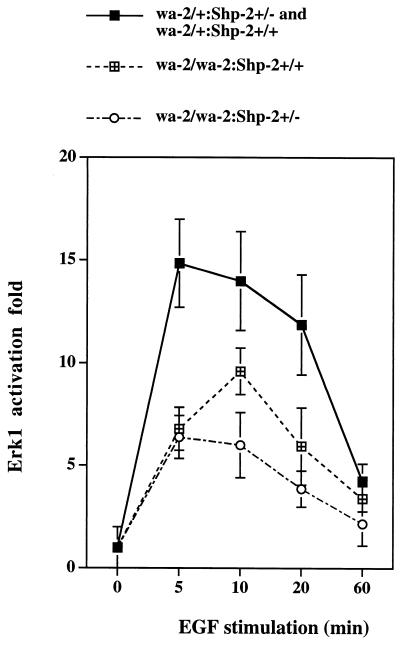
To understand the molecular basis for the genetic interaction between the wa-2 and Shp-2 mutations, we investigated the magnitude of EGF-elicited signaling in primary fibroblast cells isolated from newborns of wa-2/wa-2:Shp-2+/+, wa-2/wa-2:Shp-2+/−, and wa-2/+:Shp-2+/− or wa-2/+:Shp-2+/+ origins. Serum-starved cells were stimulated with recombinant EGF (100 ng/ml) for 5 min, and cell lysates were prepared. EGF-induced tyrosine phosphorylation of EGF-R and cytoplasmic proteins was examined (Fig. 4). Consistent with previous observations (25, 26), auto-phosphorylation of EGF-R was dramatically decreased in wa-2/wa-2 cells as compared with wa-2/+ cells, and tyrosine phosphorylation levels of Cbl, SHPS-1, and Shc also were reduced. Although the Shp-2+/− mutation did not significantly affect the wa-2 EGF-R auto-phosphorylation status, EGF-induced tyrosine phosphorylation of Cbl, SHPS-1, and Shc proteins was lower in wa-2/wa-2:Shp-2+/− than in wa-2/wa-2:Shp-2+/+ cells (Fig. 4A). A modest increase of PI3 kinase activity was induced in wa-2/+:Shp-2+/+ cells; this induction was slightly reduced in wa-2/wa-2:Shp-2+/+ cells and further decreased in wa-2/wa-2:Shp-2+/− cells (Fig. 4B). Taken together, the results suggest that a functional Shp-2 acts to propagate signals from ligand-activated EGF-R. Deficient activation of these cellular proteins in the early events of EGF-R signaling leads to reduced cellular responses to EGF that involve the stimulation of Erk activity (Fig. 5). Homozygous wa-2 mutation significantly decreased the activation of Erk1, and a heterozygous Shp-2 mutation on this background further diminishes Erk1 induction, consistent with previous biochemical data suggesting that Shp-2 enhances EGF-R signaling to Erk (17, 39, 40). Under similar conditions, Erk induction by platelet-derived growth factor or insulin-like growth factor I was not altered (data not shown), indicating a specific effect on EGF-R signaling by the wa-2 mutation.
Figure 4.
Deficient propagation of signals from EGF-R. (A) Serum-starved cells were stimulated with EGF (100 ng/ml) for 5 min, and cell lysates were prepared. Individual proteins were precipitated by their specific antibodies (αEGF-R, αCbl, αShc, and αSHPS-1) and subjected to immunoblot analysis using antiphosphotyrosine antibody (αPY). The αShc blot was stripped after αPY blotting and reprobed with αShc for loading control. Only the p66 form of Shc proteins was shown as p52 and p46 were overlapped with the IgG heavy chain. (B) PI3 kinase assay was performed by using control and EGF-stimulated cell lysates as described in the text. The experiments were repeated at least three times. Shown here are representative results.
Figure 5.
Attenuation of Erk1 activation by wa-2 and Shp-2 mutations. Primary fibroblast cells with different genotypes from newborn fetuses were established. Cells were serum-starved and subsequently stimulated by EGF (100 ng/ml) for different times as indicated. Cell lysates were prepared and subjected to Erk1 kinase assay. Mean values were derived from the data of four independent wa-2/wa-2:Shp-2+/− cell pools, three wa-2/wa-2:Shp-2+/+, and three wa-2/+:Shp-2+/− or wa-2/+:Shp-2+/+ cell pools.
DISCUSSION
In this report, we have defined Shp-2 tyrosine phosphatase as an important positive regulator of EGF-R signaling in the control of cell growth and development in mice. Involvement of Shp-2 in signal transduction downstream of a variety of receptor tyrosine kinases has been implicated by extensive biochemical data showing that Shp-2 physically interacts with growth factor receptors, such as platelet-derived growth factor receptor, EGF-R, and c-kit (41–44). Our previous phenotypic analysis of Shp-2−/− embryos suggested a critical role of Shp-2 during gastrulation in the formation of axial mesodermal and paraxial mesodermal structures (8). In this study, we pursued further analysis of Shp-2 function by creating Shp-2−/−/WT chimeric animals. This system allowed us to evaluate the Shp-2 involvement in a developmental process that otherwise would be prevented by early embryonic lethality at midgestation of Shp-2−/− embryos.
Normally, mouse eyelids close at embryonic day 16 and open again in 2 weeks after birth, a process that may be accelerated by EGF (45). Because of delayed epithelial cell development, eyelids were open at birth in EGF-R−/− mice (19, 20, 23). The prominent eye and skin abnormalities, together with the previously described immature lung development (11), in Shp-2−/−/WT animals strongly suggest that the Shp-2 mutation could specifically suppress signals from EGF-R, in addition to its other effects on mouse development. This observation prompted us to further examine the specific activity of Shp-2 in EGF-R signaling by genetic epistasis between the wa-2 and Shp-2 mutations. Our results indicate that the Shp-2 mutation dominantly enhanced the phenotype of wa-2, a weak allele of EGF-R, although Shp-2+/− mice appear normal and well developed. In this regard, it is interesting to note that a heterozygous mutation in Sos1, a guanine nucleotide exchange factor for Ras, also acts as a dominant enhancer to the wa-2 mutation (24). The combination of the wa-2/wa-2 mutation with either a Sos1+/− or a Shp-2+/− mutation resulted in an increased frequency of the open-eyelid phenotype at birth compared with that observed in wa-2 mice. Thus, the eye-defect phenotype seems to be a sensitive indicator of reduced signaling elicited by EGF-R.
Multiple pathological changes in the skin, lung, and intestine of wa-2/wa-2:Shp-2+/− mice apparently were associated with immature epithelial development, which was consistent with our previous observation that Shp-2−/− ES cells are defective in differentiation into epithelial cells in vitro (10). Remarkably, these abnormalities in tissue architecture caused by epithelial immaturity were closely related to what was seen in EGF-R−/− mice on the same C57BL/6 background (19, 20, 23).
It remains to be defined how a synergism between the wa-2 mutation and the Shp-2 mutation is achieved. A promoting effect of a heterozygous Shp-2 mutation was manifested only on a homozygous wa-2/wa-2 background, because no phenotypic difference was observed between wa-2/+:Shp-2+/+ and wa-2/+:Shp-2+/− mice. Similarly, a heterozygous Sos1 mutation enhanced the phenotype of wa-2/wa-2 but not wa-2/+ mice (24). This finding would suggest that only a homozygous mutation of wa-2 sensitizes cells for the dosage requirement of functional Shp-2 or Sos1 molecule.
The wa-2 mutation severely suppressed ligand-induced autophosphorylation of EGF-R, SHPS-1 phosphorylation, and Erk1 activation. However, phosphorylation levels of Shc proteins and the induction of PI3 kinase activity were diminished by only a small extent. The Shp-2+/− mutation under the wa-2/wa-2 background caused a further decrease in phosphorylation levels of SHPS-1, Cbl, and particularly Shc. Nevertheless, only a modest decrease of Erk1 activity was observed in wa-2/wa-2:Shp-2+/− cells as compared with wa-2/wa-2:Shp-2+/+ cells. Thus, our results would suggest a dissociation of the tyrosine phosphorylation level of Shc and PI3 kinase activity from Erk1 induction. Attenuated activation of Shc, Cbl, SHPS-1, and PI3 kinase may weaken the EGF-R signaling in several pathways, which in combination contribute to the severely deteriorating phenotype of wa-2/wa-2:Shp-2+/− mice. Although the mechanism is not known, Shp-2 tyrosine phosphatase apparently acts to promote the tyrosine phosphorylation of Cbl, SHPS-1, and Shc in EGF-treated cells, possibly by stimulating a receptor-coupled kinase(s), such as a Src family member. c-Src was shown to be required for EGF-R signaling (46), and Shp-2 physically interacts with c-Src and dephosphorylates Tyr-527 at the C-terminal tail of Src in vitro, a major mechanism known to up-regulate Src activity (47).
A recent report suggested that Csw, the Drosophila homologue of Shp-2, positively modulates the strength of signals from Torso by dephosphorylating a RasGAP binding site on Torso (48). Tyrosine-phosphorylated Csw (Shp-2) also may act as an adaptor linking a receptor tyrosine kinase to the Ras pathway via interaction with Drk (Grb2). However, Shp-2 is not phosphorylated on tyrosine in response to EGF stimulation unless the EGF-R is overexpressed (ref. 41 and data not shown). In previous experiments, we failed to detect a significant dephosphorylation effect of Shp-2 on EGF-R (41). Further investigation is needed to understand the role of Shp-2 in signaling events downstream of growth factor receptors and integrins.
Although a mutant Shp-2 protein with an internal deletion of the SH2-N domain was expressed from the targeted mutant allele, it seems to be unlikely that the mutant phenotype is the result of an unregulated phosphatase activity. So far, the molecular and developmental defects observed in our Shp-2−/− cells and animals are remarkably similar to what were seen in mammalian cells and Xenopus embryos with ectopic expression of a catalytically inactive mutant of Shp-2. Therefore, without the intact SH2-N domain, the phosphatase might not be able to properly target to the substrate(s). It is also conceivable that the much reduced expression level of the mutant protein may contribute to the defective phenotype, even if it had some residual biological activity. The best way to resolve this issue is to generate a Shp-2 null mutant mouse model and to compare its phenotype with that of SH2-N deletion mutant.
Acknowledgments
We thank Drs. Howard Edenberg, Mark Kaplan, Alexander Dent, and other members of our laboratory for critical reading of the manuscript and helpful discussions. This work was supported by grants from the National Institutes of Health (R29GM53660 and R01CA78606) (to G.-S.F.). G.-S.F. had a career development award from American Diabetes Association.
ABBREVIATIONS
- EGF
epidermal growth factor
- EGF-R
EGF receptor
- SH2
Src homology 2
- SH2-N
N-terminal SH2
- ES
embryonic stem
- WT
wild type
- PI3
phosphoinositide 3
- Erk
extracellular signal-regulated kinase
- SHPS
Src homology-containing phosphatase substrate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Feng G-S, Pawson T. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 2.Neel B G, Tonks N K. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 3.Perkins L A, Larsen I, Perrimon N. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 4.Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. Development (Cambridge, UK) 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- 5.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 6.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 7.Tang T L, Freeman R, Jr, O’Reilly A M, Neel B G, Sokol S Y. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 8.Saxton T M, Henkemeyer M, Gasca S, Shen R, Shalaby F, Feng G-S, Pawson T. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu C-K, Shi Z Q, Shen R, Tsai F Y, Orkin S H, Feng G-S. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu C-K, Feng G-S. Oncogene. 1998;17:433–440. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- 11.Qu C-K, Yu W-M, Azzarelli B, Cooper S, Broxmeyer H E, Feng G-S. Mol Cell Biol. 1998;18:6075–6082. doi: 10.1128/mcb.18.10.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlowski M, Mlinaric-Rascan I, Feng G-S, Shen R, Pawson T, Siminovitch K A. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shultz L D, Schweitzer P A, Rajan T V, Yi T, Ihle J N, Matthews R J, Thomas M L, Beier D R. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 14.Tsui H W, Siminovitch K A, de Souza L, Tsui F W. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 15.Tsui F W, Tsui H W. Immunol Rev. 1994;138:185–206. doi: 10.1111/j.1600-065x.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z Q, Lu W, Feng G-S. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 18.Yu D H, Qu C-K, Henegariu O, Lu X, Feng G-S. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen P J, Berger J E, Meneses J, Phung Y, Pedersen R A, Werb Z, Derynck R. Nature (London) 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 20.Sibilia M, Wagner E F. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 21.Luetteke N C, Qiu T H, Peiffer R L, Oliver P, Smithies O, Lee D C. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 22.Mann G B, Fowler K J, Gabriel A, Nice E C, Williams R L, Dunn A R. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 23.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 24.Wang D Z, Hammond V E, Abud H E, Bertoncello I, McAvoy J W, Bowtell D D. Genes Dev. 1997;11:309–320. doi: 10.1101/gad.11.3.309. [DOI] [PubMed] [Google Scholar]
- 25.Fowler K J, Walker F, Alexander W, Hibbs M L, Nice E C, Bohmer R M, Mann G B, Thumwood C, Maglitto R, Danks J A, et al. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luetteke N C, Phillips H K, Qiu T H, Copeland N G, Earp H S, Jenkins N A, Lee D C. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 27.Crew F A E. J Genet. 1993;27:633–646. [Google Scholar]
- 28.Oh W, Stern L. In: Neonatal Medicine. Stern L, Vert P, editors. New York: Masson; 1987. p. 395. [Google Scholar]
- 29.van der Geer P, Hunter T, Lindberg R A. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 30.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci P G, et al. Nature (London) 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 31.Pronk G J, de Vries-Smits A M, Buday L, Downward J, Maassen J A, Medema R H, Bos J L. Mol Cell Biol. 1994;14:1575–1581. doi: 10.1128/mcb.14.3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downward J. Cancer Surv. 1996;27:87–100. [PubMed] [Google Scholar]
- 33.Ochi F, Matozaki T, Noguchi T, Fujioka Y, Yamao T, Takada T, Tsuda M, Takeda H, Fukunaga K, Okabayashi Y, Kasuga M. Biochem Biophys Res Commun. 1997;239:483–487. doi: 10.1006/bbrc.1997.7489. [DOI] [PubMed] [Google Scholar]
- 34.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. Nature (London) 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 36.Fukazawa T, Miyake S, Band V, Band H. J Biol Chem. 1996;271:14554–14559. doi: 10.1074/jbc.271.24.14554. [DOI] [PubMed] [Google Scholar]
- 37.Meisner H, Czech M P. J Biol Chem. 1995;270:25332–25335. doi: 10.1074/jbc.270.43.25332. [DOI] [PubMed] [Google Scholar]
- 38.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 39.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Neel B G. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deb T B, Wong L, Salomon D S, Zhou G, Dixon J E, Gutkind J S, Thompson S A, Johnson G R. J Biol Chem. 1998;273:16643–16646. doi: 10.1074/jbc.273.27.16643. [DOI] [PubMed] [Google Scholar]
- 41.Feng G-S, Hui C C, Pawson T. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 42.Vogel W, Lammers R, Huang J, Ullrich A. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 43.Lechleider R J, Freeman R, Jr, Neel B G. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 44.Tauchi T, Feng G-S, Marshall M S, Shen R, Mantel C, Pawson T, Broxmeyer H E. J Biol Chem. 1994;269:25206–25211. [PubMed] [Google Scholar]
- 45.Cohen S. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 46.Broome M A, Hunter T. J Biol Chem. 1996;271:16798–16806. doi: 10.1074/jbc.271.28.16798. [DOI] [PubMed] [Google Scholar]
- 47.Peng Z Y, Cartwright C A. Oncogene. 1995;11:1955–1962. [PubMed] [Google Scholar]
- 48.Cleghon V, Feldmann P, Ghiglione C, Copeland T D, Perrimon N, Hughes D A, Morrison D K. Mol Cell. 1998;2:719–727. doi: 10.1016/s1097-2765(00)80287-7. [DOI] [PubMed] [Google Scholar]