Abstract
Background: Galectin-3 is a lectin detected in mature and early hypertrophic chondrocytes; osteoarthritic (OA) chondrocytes can re-express hypertrophic markers.
Objective: To investigate the synthesis and subcellular localisation of galectin-3 in adult chondrocytes as well as the possibility of cleavage of galectin-3 by collagenase-1 and -3.
Methods: Galectin-3 was assessed by immunohistochemistry and real time polymerase chain reaction (PCR) in normal and OA cartilage. Its localisation was investigated by subcellular fractionation, immunocytology, and flow cytometry. Proteolysis of galectin-3 by collagenase-1 and -3 was determined by in vitro assay.
Results: Galectin-3 expression was increased 2.4-fold as measured by reverse transcriptase (RT)-PCR (p<0.05, n = 5) and threefold by immunohistochemistry (p<0.003 n = 6) in OA cartilage compared with normal cartilage. In adult chondrocytes, galectin-3 was found in the cytosol and membrane enriched fractions. Both immunocytology and flow cytometry confirmed the presence of galectin-3 at the surface of chondrocytes. A strong correlation was found between integrin-ß1 and galectin-3 expression at the surface of chondrocytes. Moreover, collagenase-3 cleaved galectin-3 with a higher activity than collagenase-1. The proteolysed sites generated were identical to those produced by gelatinases A and B.
Conclusion: Galectin-3 may play a part in OA, having two roles, one intracellular and not yet identified, and another at the cell surface, possibly related to the interaction of chondrocytes and the cartilage matrix.
Full Text
The Full Text of this article is available as a PDF (257.0 KB).
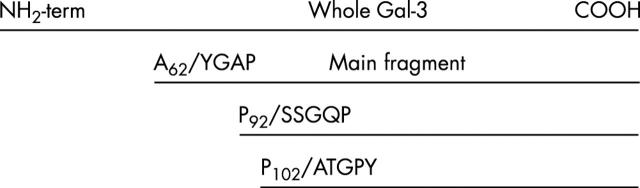
Figure 1 .
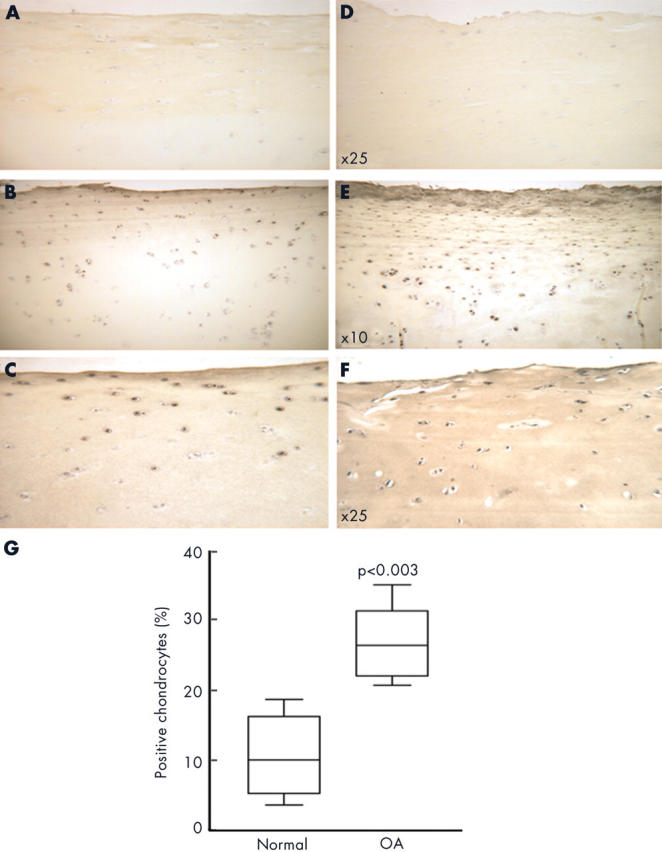
Representative section of cartilage, showing immunostaining for galectin-3 from normal (A–C) and OA cartilage (D–F). (A and D) Non-immune serum; (B, C, E, and F) polyclonal anti-galectin-3. Original magnification x25; (G) galectin-3 levels over the entire cartilage thickness. Data are presented as box plots, where the boxes represent the first and third quartiles, the lines within the boxes represent the median, and the lines outside the boxes represent the spread of the galectin-3 cell scores outside the first and third quartiles. The p value, obtained with the Mann-Whitney test, indicates the difference compared with the normal group.
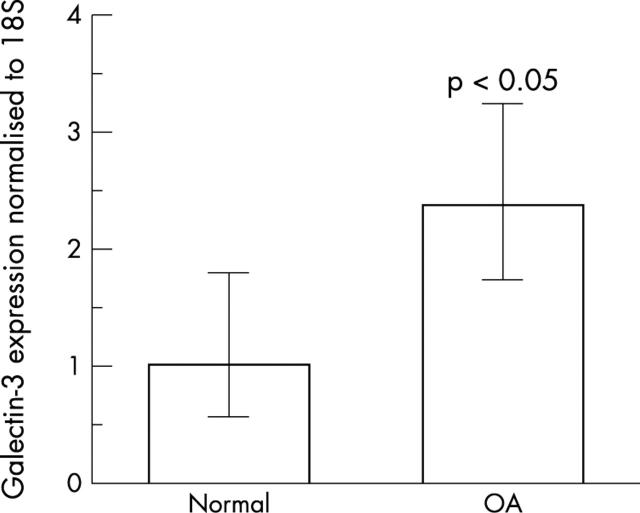
Figure 2 .
Expression of galectin-3 mRNA in human normal and OA cartilage was studied using real time RT-PCR as described in "Materials and methods". The PCR analysis was performed by normalising the PCR products of the galectin-3 to the 18S PCR products. Bars show the mean (SD) intensity of five normal and OA specimens. The p value, obtained with Student's t test, indicates the difference compared with the normal group.
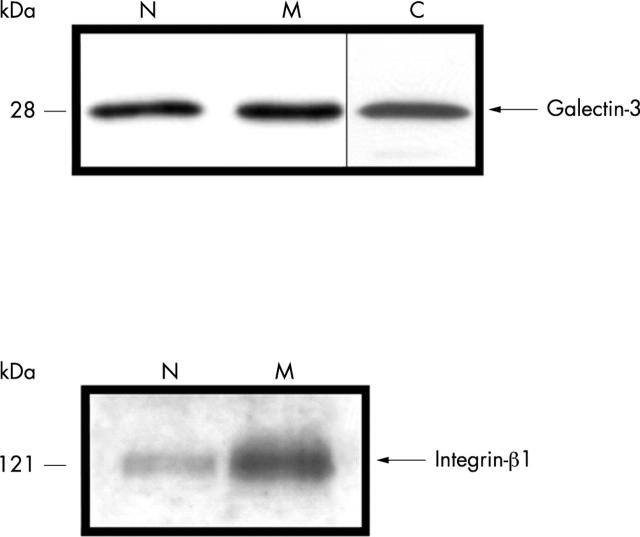
Figure 3 .
Subcellular OA chondrocyte fractionation was performed as described in "Materials and methods". Proteins were quantified for each fraction, 10 µg were blotted, and immunodetection was performed with either a polyclonal anti-galectin-3 or a monoclonal anti-integrin-ß1 antibody. N, nuclear fraction; M, membrane enriched fraction; C, cytosol.
Figure 4 .
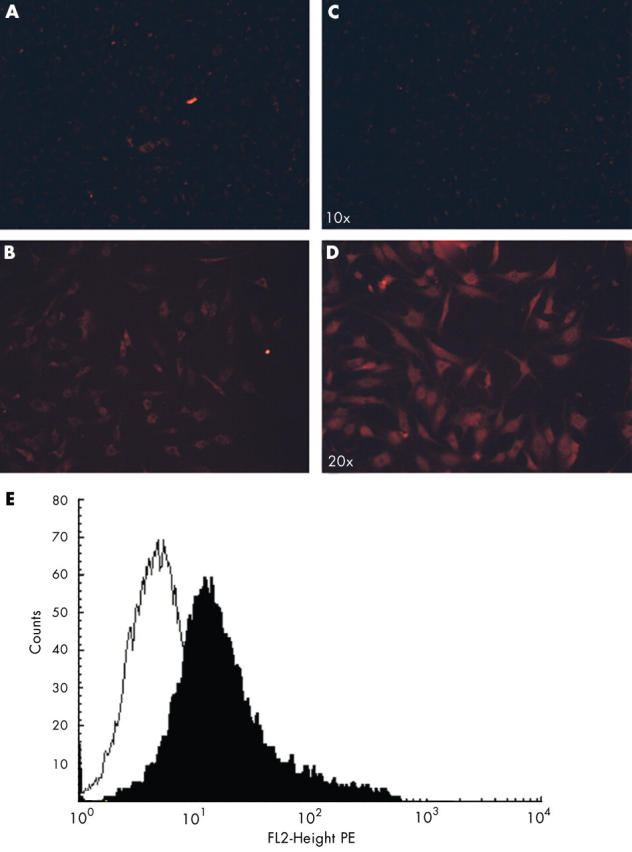
Immunofluorescence and flow cytometry detection of galectin-3 in OA chondrocytes. Cells were incubated with a specific anti-galectin-3 polyclonal antibody (B, D) or non-immune-serum (A, C) and detected with a fluorescent conjugated secondary antibody. (A, B) non-permeabilised cells; (C, D) permeabilised cells; (E) cell surface expression of galectin-3 by primary OA articular chondrocytes. Non-permeabilised cells were immunostained with a polyclonal anti-galectin-3 (filled histogram) or with non-immune serum (open histogram) followed by phycoerythrin (PE) conjugated secondary antibody. The assessment that chondrocytes were not permeabilised was performed by using the 7-AAD dye, which penetrates into the cells, only when the membrane is disrupted.
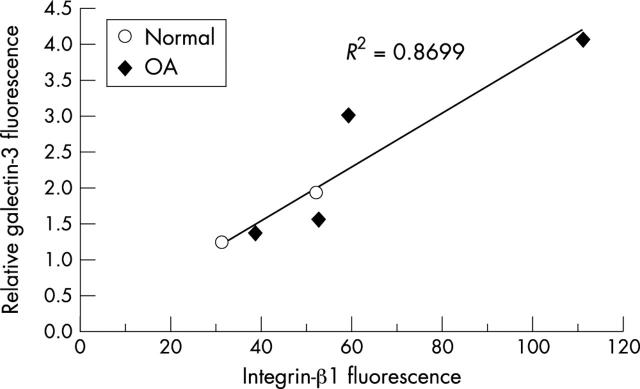
Figure 5 .
Correlation of galectin-3 presence with integrin-ß1 at the chondrocyte surface. Cell surface fluorescence was measured with either galectin-3 serum or integrin-ß1 antibody on non-permeabilised cells, as explained in "Materials and methods". As non-immune serum produced background level discrepancies, galectin-3 fluorescence was normalised to the non-immune signal. Relative galectin-3 fluorescence = galectin-3 fluorescence divided by non-immune fluorescence.
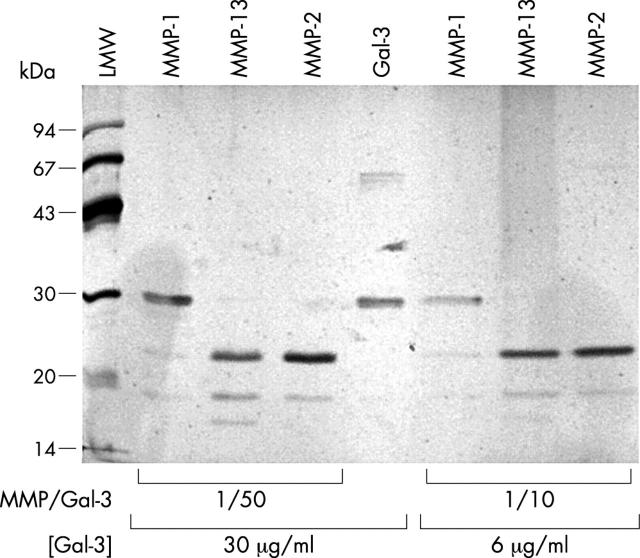
Figure 6 .
Proteolytic activity of collagenase-3 (MMP-13) on galectin-3. rh-galectin-3 was incubated in the presence of activated MMPs as described in "Materials and methods". As galectin-3 at high concentration could multimerise,26 two galectin-3 concentrations were used: 30 µg/ml (multimerisation) and 6 µg/ml (monomerisation). The reaction mixture was stopped with a solution of 6xLaemmli buffer, loaded onto an SDS-PAGE system, and the galectin-3 was stained with silver nitrate. MMP-1, collagenase-1; MMP-13, collagenase-3; MMP-2, gelatinase A; Gal-3, galectin-3.
Figure 7 .
Schematic representation of galectin-3 sites generated by collagenase-3.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahani S., Nangia-Makker P., Inohara H., Kim H. R., Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997 Dec 1;57(23):5272–5276. [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Liu F., Malaval L., Gupta A. K. Osteoblast and chondroblast differentiation. Bone. 1995 Aug;17(2 Suppl):77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- Bao Q., Hughes R. C. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995 Aug;108(Pt 8):2791–2800. doi: 10.1242/jcs.108.8.2791. [DOI] [PubMed] [Google Scholar]
- Barondes S. H. Bifunctional properties of lectins: lectins redefined. Trends Biochem Sci. 1988 Dec;13(12):480–482. doi: 10.1016/0968-0004(88)90235-6. [DOI] [PubMed] [Google Scholar]
- Boileau Christelle, Martel-Pelletier Johanne, Moldovan Florina, Jouzeau Jean-Yves, Netter Patrick, Manning Pamela T., Pelletier Jean-Pierre. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002 Oct;46(10):2637–2647. doi: 10.1002/art.10518. [DOI] [PubMed] [Google Scholar]
- Burton-Wurster N., Butler M., Harter S., Colombo C., Quintavalla J., Swartzendurber D., Arsenis C., Lust G. Presence of fibronectin in articular cartilage in two animal models of osteoarthritis. J Rheumatol. 1986 Feb;13(1):175–182. [PubMed] [Google Scholar]
- Campbell C. J. The healing of cartilage defects. Clin Orthop Relat Res. 1969 May-Jun;64:45–63. [PubMed] [Google Scholar]
- Colnot C., Sidhu S. S., Balmain N., Poirier F. Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev Biol. 2001 Jan 1;229(1):203–214. doi: 10.1006/dbio.2000.9933. [DOI] [PubMed] [Google Scholar]
- Colnot C., Sidhu S. S., Poirier F., Balmain N. Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell Mol Biol (Noisy-le-grand) 1999 Dec;45(8):1191–1202. [PubMed] [Google Scholar]
- Craig S. S., Krishnaswamy P., Irani A. M., Kepley C. L., Liu F. T., Schwartz L. B. Immunoelectron microscopic localization of galectin-3, an IgE binding protein, in human mast cells and basophils. Anat Rec. 1995 Jun;242(2):211–219. doi: 10.1002/ar.1092420210. [DOI] [PubMed] [Google Scholar]
- Dagher S. F., Wang J. L., Patterson R. J. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg L., Billinghurst R. C., Manner P., Nelson F., Webb G., Ionescu M., Reiner A., Tanzer M., Zukor D., Chen J. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1). Arthritis Rheum. 2000 Mar;43(3):673–682. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Hughes R. C. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen). Glycoconj J. 1997 Feb;14(2):267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- Enomoto M., Leboy P. S., Menko A. S., Boettiger D. Beta 1 integrins mediate chondrocyte interaction with type I collagen, type II collagen, and fibronectin. Exp Cell Res. 1993 Apr;205(2):276–285. doi: 10.1006/excr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Fowlis D., Colnot C., Ripoche M. A., Poirier F. Galectin-3 is expressed in the notochord, developing bones, and skin of the postimplantation mouse embryo. Dev Dyn. 1995 Jun;203(2):241–251. doi: 10.1002/aja.1002030211. [DOI] [PubMed] [Google Scholar]
- Frigeri L. G., Zuberi R. I., Liu F. T. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993 Aug 3;32(30):7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- Hsu D. K., Zuberi R. I., Liu F. T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992 Jul 15;267(20):14167–14174. [PubMed] [Google Scholar]
- Hubert M., Wang S. Y., Wang J. L., Sève A. P., Hubert J. Intranuclear distribution of galectin-3 in mouse 3T3 fibroblasts: comparative analyses by immunofluorescence and immunoelectron microscopy. Exp Cell Res. 1995 Oct;220(2):397–406. doi: 10.1006/excr.1995.1331. [DOI] [PubMed] [Google Scholar]
- Kadrofske M. M., Openo K. P., Wang J. L. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998 Jan 1;349(1):7–20. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- Kim H. K., Moran M. E., Salter R. B. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am. 1991 Oct;73(9):1301–1315. [PubMed] [Google Scholar]
- Kim H. R., Lin H. M., Biliran H., Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999 Aug 15;59(16):4148–4154. [PubMed] [Google Scholar]
- Kirsch T., Swoboda B., Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000 Jul;8(4):294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996 Jan 19;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Kurtis Melissa S., Schmidt Tannin A., Bugbee William D., Loeser Richard F., Sah Robert L. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheum. 2003 Jan;48(1):110–118. doi: 10.1002/art.10704. [DOI] [PubMed] [Google Scholar]
- Loeser R. F., Carlson C. S., McGee M. P. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res. 1995 Apr;217(2):248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- Loeser R. F., Sadiev S., Tan L., Goldring M. B. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000 Mar;8(2):96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- Mitchell P. G., Magna H. A., Reeves L. M., Lopresti-Morrow L. L., Yocum S. A., Rosner P. J., Geoghegan K. F., Hambor J. E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996 Feb 1;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Hambor J., Cloutier J. M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997 Sep;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., Mok M. T., King K. B., Gupta M., Chubinskaya S., Koepp H., Cole A. A. Expression of anchorin CII (cartilage annexin V) in human young, normal adult, and osteoarthritic cartilage. J Histochem Cytochem. 1999 Feb;47(2):209–220. doi: 10.1177/002215549904700209. [DOI] [PubMed] [Google Scholar]
- Moutsatsos I. K., Davis J. M., Wang J. L. Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. J Cell Biol. 1986 Feb;102(2):477–483. doi: 10.1083/jcb.102.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlich A. G., Schleicher E. D., Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine (Phila Pa 1976) 1997 Dec 15;22(24):2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- Nurminskaya M., Linsenmayer T. F. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev Dyn. 1996 Jul;206(3):260–271. doi: 10.1002/(SICI)1097-0177(199607)206:3<260::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Fridman R., Nangia-Makker P., Kleiner D. E., Liotta L. A., Stetler-Stevenson W. G., Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994 Nov 29;33(47):14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Green B., Evans S., James O., Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998 Jan 8;1379(1):97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Leite-Browning M. L., Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem Biophys Res Commun. 1998 May 29;246(3):788–791. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Platt D., Tait L., Hogan V., Raz T., Carmi P., Raz A. Structure-function relationship of a recombinant human galactoside-binding protein. Biochemistry. 1993 Apr 27;32(16):4455–4460. doi: 10.1021/bi00067a038. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Warfield P., Green-Jarvis B., Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem. 1999 Dec 1;75(3):505–514. doi: 10.1002/(sici)1097-4644(19991201)75:3<505::aid-jcb14>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Lascau-Coman V., Jovanovic D., Fernandes J. C., Manning P., Connor J. R., Currie M. G., Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J Rheumatol. 1999 Sep;26(9):2002–2014. [PubMed] [Google Scholar]
- Piperno M., Reboul P., Hellio le Graverand M. P., Peschard M., Annefeld M., Richard M., Vignon E. Osteoarthritic cartilage fibrillation is associated with a decrease in chondrocyte adhesion to fibronectin. Osteoarthritis Cartilage. 1998 Nov;6(6):393–399. doi: 10.1053/joca.1998.0138. [DOI] [PubMed] [Google Scholar]
- Probstmeier R., Montag D., Schachner M. Galectin-3, a beta-galactoside-binding animal lectin, binds to neural recognition molecules. J Neurochem. 1995 Jun;64(6):2465–2472. doi: 10.1046/j.1471-4159.1995.64062465.x. [DOI] [PubMed] [Google Scholar]
- Pulai Judit I., Del Carlo Marcello, Jr, Loeser Richard F. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002 Jun;46(6):1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- Pullig O., Weseloh G., Gauer S., Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol. 2000 Jul;19(3):245–255. doi: 10.1016/s0945-053x(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Raimond J., Zimonjic D. B., Mignon C., Mattei M., Popescu N. C., Monsigny M., Legrand A. Mapping of the galectin-3 gene (LGALS3) to human chromosome 14 at region 14q21-22. Mamm Genome. 1997 Sep;8(9):706–707. doi: 10.1007/s003359900548. [DOI] [PubMed] [Google Scholar]
- Raz A., Pazerini G., Carmi P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 1989 Jul 1;49(13):3489–3493. [PubMed] [Google Scholar]
- Reboul P., Pelletier J. P., Tardif G., Benderdour M., Ranger P., Bottaro D. P., Martel-Pelletier J. Hepatocyte growth factor induction of collagenase 3 production in human osteoarthritic cartilage: involvement of the stress-activated protein kinase/c-Jun N-terminal kinase pathway and a sensitive p38 mitogen-activated protein kinase inhibitor cascade. Arthritis Rheum. 2001 Jan;44(1):73–84. doi: 10.1002/1529-0131(200101)44:1<73::AID-ANR11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Reboul P., Pelletier J. P., Tardif G., Cloutier J. M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996 May 1;97(9):2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992 Apr 5;267(10):6983–6990. [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Control of Mac-2 surface expression on murine macrophage cell lines. Eur J Immunol. 1994 Jan;24(1):216–221. doi: 10.1002/eji.1830240134. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Van den Brûle F. A., Fernandez P. L., Buicu C., Liu F. T., Jackers P., Lambotte R., Castronovo V. Differential expression of galectin-1 and galectin-3 during first trimester human embryogenesis. Dev Dyn. 1997 Aug;209(4):399–405. doi: 10.1002/(SICI)1097-0177(199708)209:4<399::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Verzijl Nicole, DeGroot Jeroen, Ben Zaken Chaya, Brau-Benjamin Orit, Maroudas Alice, Bank Ruud A., Mizrahi Joe, Schalkwijk Casper G., Thorpe Suzanne R., Baynes John W. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002 Jan;46(1):114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Li Y. M., Imani F., Wojciechowicz D., Yang Z., Liu F. T., Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995 Sep;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- Wang L., Inohara H., Pienta K. J., Raz A. Galectin-3 is a nuclear matrix protein which binds RNA. Biochem Biophys Res Commun. 1995 Dec 5;217(1):292–303. doi: 10.1006/bbrc.1995.2777. [DOI] [PubMed] [Google Scholar]
- Yang R. Y., Hsu D. K., Liu F. T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brûle F. A., Buicu C., Sobel M. E., Liu F. T., Castronovo V. Galectin-3, a laminin binding protein, fails to modulate adhesion of human melanoma cells to laminin. Neoplasma. 1995;42(5):215–219. [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]