Abstract
Objectives: To better understand the role of collagenase-3 (MMP-13) in joint inflammation by investigating the consequences of transient overexpression of human collagenase-3 (matrix metalloproteinase-13 (MMP-13)), introduced by adenoviral gene delivery, in the mouse knee joint.
Methods: A single dose (5x107 pfu) of recombinant adenovirus coding either for ß-galactosidase (RAdLacZ) or human MMP-13 (RAdMMP-13) was injected intra-articularly into the knee joint of adult mice. The joints were analysed at frequent intervals up to 4 weeks by histology, immunohistochemistry, and RNA analysis.
Results: When RAdLacZ reporter virus was used, adenoviruses efficiently infected synovial cells, chondrocytes of articular cartilage, and hypertrophic chondrocytes of the growth plate. The infection was transient as no reporter gene activity was detected 3 weeks after the injection. After RAdMMP-13 injection into the knee joints, expression of human MMP-13 in joint tissues resulted in an arthritis characterised by recruitment of inflammatory cells and increased production of cytokines and chemokines, synovial hyperplasia, and pannus formation. After the loss of MMP-13 transgene expression at 3 weeks, these inflammatory changes began to diminish.
Conclusions: MMP-13 has a role in the onset of inflammatory reaction in synovium. However, damage to articular cartilage was only rarely detected after the short term overexpression of MMP-13.
Full Text
The Full Text of this article is available as a PDF (625.4 KB).
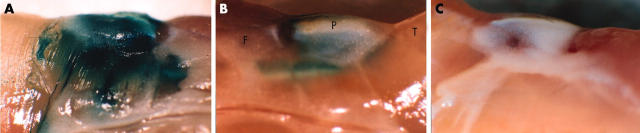
Figure 1 .
The in vivo transduction efficiency of RAdLacZ. Lateral view of mouse knee joints (A) 1 and (B) 2 weeks after intra-articular injection of 5x107 pfu of RAdLacZ. The joint was dissected as a block, fixed, and stained with X-Gal. The contralateral, uninjected knee exhibited no ß-galactosidase activity as shown 1 week after the injection (C). P, patella; T, tibial muscles; F, femoral muscles.
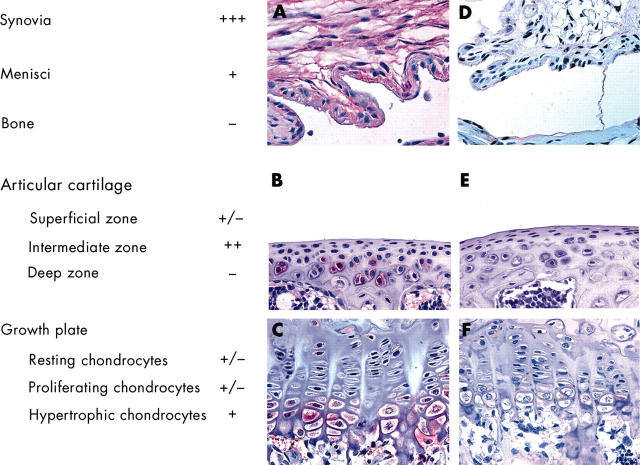
Figure 2 .
Recombinant adenoviruses infect different tissues and cells in the knee joint. Immunohistochemical localisation of ß-galactosidase in mouse knee joints using a monoclonal antibody was performed 1 week after injection with either RAdLacZ (A, B, and C) or a control virus RAd66 (D, E, and F). Representative sections of synovium (A and D), articular cartilage (B and E), and growth plate (C and F) are shown. These immunohistochemical analyses were also used to measure the transduction efficiency of RAdLacZ to various cells and tissues of the knee joint. Scoring shown to the left is based on the percentage of the cells positive for ß-galactosidase: –, no positive cells; +/–, 1–24%; +, 25–49%; ++, 50–74%; +++, 75–100%.
Figure 3 .
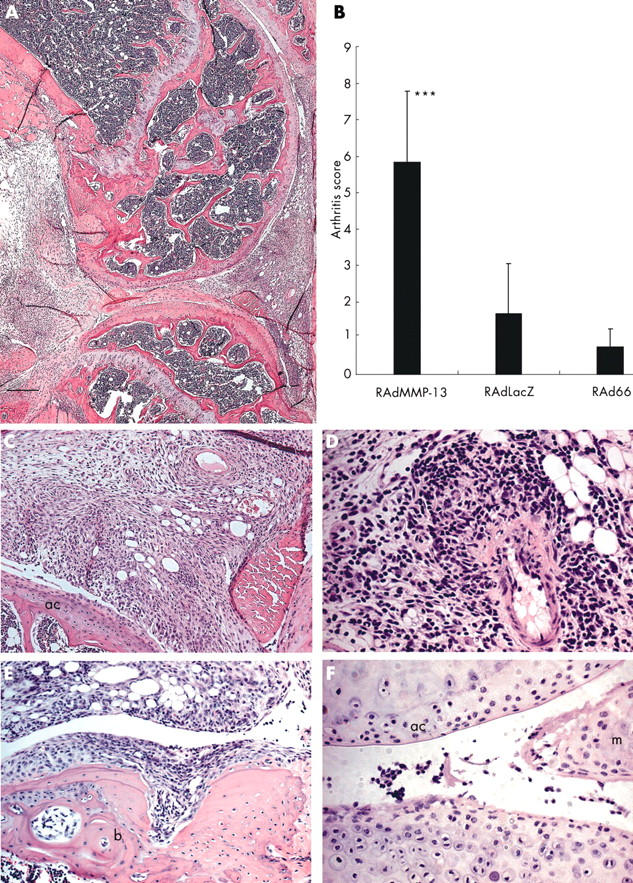
Overexpression of MMP-13 results in inflammatory synovitis. Histopathology of RAdMMP-13 treated mouse knee joints 1 week after injection. (A) Photomicrograph of a sagittal section of the inflamed knee joint; (B) qualitative analysis of synovitis based on synovial hyperplasia, fibrosis, inflammation, and necrosis in the RAdMMP-13, RAdLacZ, and RAd66 injected knees (n = 5–6 in each group). Values are the mean (SD); (C) synovial hyperplasia; (D) inflammatory reaction centre consisting of mononuclear cells; (E) invasive pannus formation; (F) superficial erosion of articular cartilage and meniscus were found in the RAdMMP-13 injected joints. The sections were counterstained with haematoxylin and the scale bar represents 800 µm (panel A), 200 µm (panels C and E), and 100 µm (for panels D and F). ac, articular cartilage; b, bone; m, meniscus.
Figure 4 .
RAdMMP-13 injection increased MMP-13 mRNA production in the knee joint. (A) RT-PCR analysis of human MMP-13 mRNAs in RAdMMP-13 injected knee joints. RNA extracted from a squamous cellular carcinoma sample was used as a positive control. Aliquots of RNA extracted 3, 7, and 21 days after the injection were used for cDNA synthesis by reverse transcriptase reaction and subsequent amplification by PCR. The reaction products were electrophoresed on a 1% agarose gel with molecular weight markers. The expected human MMP-13 derived band of 300 bp is seen migrating close to the 300 bp fragment of the standard. (B) Compiled data from northern analysis of total RNAs extracted from intact, RAdLacZ, or RAdMMP-13 injected knee joints at 1 and 3 weeks after injection (n = 6 at each time) using a probe for murine MMP-13 and 28S ribosomal RNA. The hybridisation intensity was analysed with a phosphoimager and normalised to a constant amount of 28S RNA. Values are mean (SD). Northern analysis: *p<0.05.
Figure 5 .
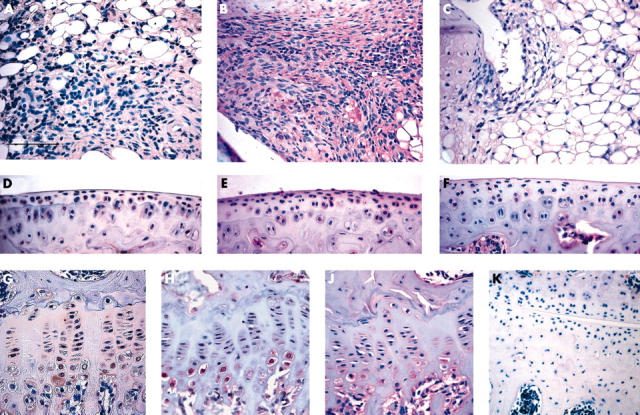
Immunohistochemical localisation of MMP-13 production in the joint. Immunohistochemical localisation of human MMP-13 production in the synovium (A), articular cartilage (D), and growth plate (G) 1 week after injection of RAdMMP-13 using a monoclonal antibody recognising only human MMP-13. Binding of the antibody was detected as a reddish brown precipitate using the avidin-biotin complex method. As a control (K) the monoclonal antibody against human MMP-13 was also applied on a section of uninjected control knee. Localisation of both human and mouse MMP-13 by immunohistochemistry in joint tissues 1 week after injection of RAdMMP-13 (B, E, and H) or in uninjected knee joints (C, F, and J) using polyclonal antibody (B and C synovium, E and F articular cartilage, H and J growth plate). Binding of antibodies was detected using biotin labelled secondary antibody and alkaline phosphatase conjugated streptavidin. The sections were counterstained with haematoxylin. The scale bar in panel A represents 100 µm for panel K and 50 µm for the other panels.
Figure 6 .
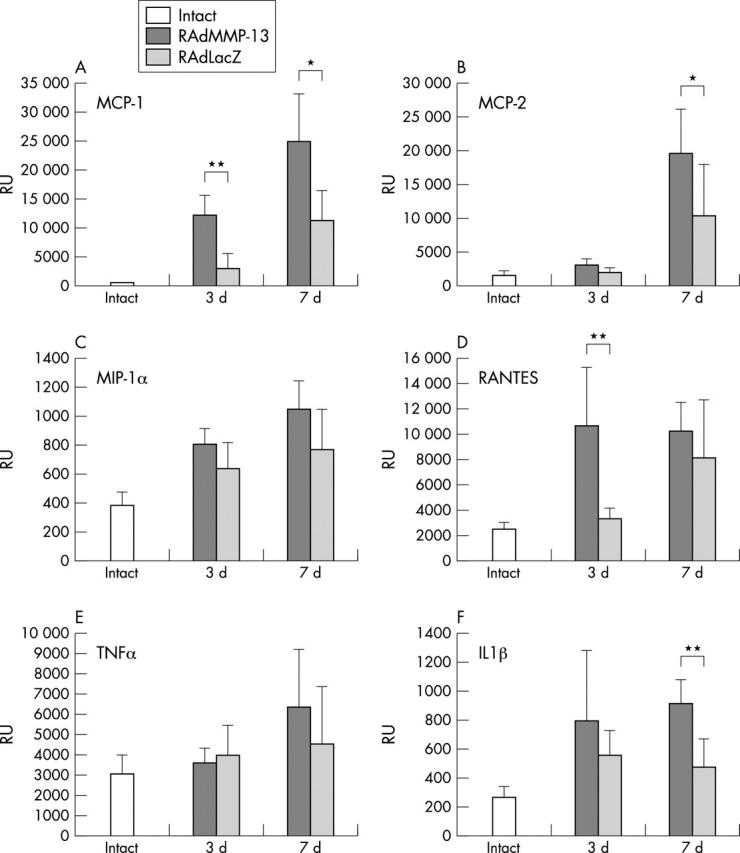
Expression of chemokines and cytokines is up regulated after RAdMMP-13 injection Production of mRNAs for (A) MCP-1, (B) MCP-2, (C) MIP-1α, (D) RANTES, (E) TNFα, and (F) IL1ß mRNA production was measured in intact, RAdMMP-13, and RAdLacZ injected mouse knee joints (n = 5–6 per group) using a quantitative Taqman RT-PCR method. The results were normalised for a constant amount of 18S ribosomal RNA. For statistical analyses a Wilcoxson matched pair test was used: *p<0.05, **p<0.005 significance of mRNA levels in RAdMMP-13 treated knee joints over those injected with RAdLacZ.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Aho Risto, Johansson Nina, Baker Andrew H., Kähäri Veli-Matti. Expression of collagenase-3 (MMP-13) enhances invasion of human fibrosarcoma HT-1080 cells. Int J Cancer. 2002 Jan 20;97(3):283–289. doi: 10.1002/ijc.1619. [DOI] [PubMed] [Google Scholar]
- Ala-Aho Risto, Johansson Nina, Baker Andrew H., Kähäri Veli-Matti. Expression of collagenase-3 (MMP-13) enhances invasion of human fibrosarcoma HT-1080 cells. Int J Cancer. 2002 Jan 20;97(3):283–289. doi: 10.1002/ijc.1619. [DOI] [PubMed] [Google Scholar]
- Alaaeddine N., Olee T., Hashimoto S., Creighton-Achermann L., Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001 Jul;44(7):1633–1643. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Arai Y., Kubo T., Kobayashi K., Takeshita K., Takahashi K., Ikeda T., Imanishi J., Takigawa M., Hirasawa Y. Adenovirus vector-mediated gene transduction to chondrocytes: in vitro evaluation of therapeutic efficacy of transforming growth factor-beta 1 and heat shock protein 70 gene transduction. J Rheumatol. 1997 Sep;24(9):1787–1795. [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Billinghurst R. C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997 Apr 1;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Panayi G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Conti Pio, Reale Marcella, Barbacane Renato C., Castellani Maria Luisa, Orso Claudio. Differential production of RANTES and MCP-1 in synovial fluid from the inflamed human knee. Immunol Lett. 2002 Feb 1;80(2):105–111. doi: 10.1016/s0165-2478(01)00303-0. [DOI] [PubMed] [Google Scholar]
- Engelholm L. H., Nielsen B. S., Danø K., Behrendt N. The urokinase receptor associated protein (uPARAP/endo180): a novel internalization receptor connected to the plasminogen activation system. Trends Cardiovasc Med. 2001 Jan;11(1):7–13. doi: 10.1016/s1050-1738(01)00076-7. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Maini R. N. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Haro H., Crawford H. C., Fingleton B., MacDougall J. R., Shinomiya K., Spengler D. M., Matrisian L. M. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000 Jan;105(2):133–141. doi: 10.1172/JCI7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro H., Crawford H. C., Fingleton B., Shinomiya K., Spengler D. M., Matrisian L. M. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000 Jan;105(2):143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999 Oct 1;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Ibrahim Saleh Mohamed, Koczan Dirk, Thiesen Hans-Jürgen. Gene-expression profile of collagen-induced arthritis. J Autoimmun. 2002 Mar;18(2):159–167. doi: 10.1006/jaut.2001.0580. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kubo T., Arai Y., Nakanishi T., Kobayashi K., Takahashi K., Imanishi J., Takigawa M., Hirasawa Y. Adenovirus mediated gene delivery to the joints of guinea pigs. J Rheumatol. 1998 Sep;25(9):1666–1673. [PubMed] [Google Scholar]
- Johansson N., Saarialho-Kere U., Airola K., Herva R., Nissinen L., Westermarck J., Vuorio E., Heino J., Kähäri V. M. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev Dyn. 1997 Mar;208(3):387–397. doi: 10.1002/(SICI)1097-0177(199703)208:3<387::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Ainola M., Valleala H., Ma J., Ida H., Mandelin J., Kinne R. W., Santavirta S., Sorsa T., López-Otín C. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999 Nov;58(11):691–697. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen Y. T., Salo T., Hanemaaijer R., Valleala H., Sorsa T., Sutinen M., Ceponis A., Xu J. W., Santavirta S., Teronen O. Collagenase-3 (MMP-13) and its activators in rheumatoid arthritis: localization in the pannus-hard tissue junction and inhibition by alendronate. Matrix Biol. 1999 Aug;18(4):401–412. doi: 10.1016/s0945-053x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Petulant cellular acts: destroying the ECM rather than creating it. J Clin Invest. 2001 Jan;107(1):31–32. doi: 10.1172/JCI11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi E., Fridman R., Miao H. Q., Ma Y. S., Yayon A., Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. B., Flannery C. R., Hughes C. E., Mort J. S., Roughley P. J., Dent C., Caterson B. Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem J. 1999 Nov 15;344(Pt 1):61–68. [PMC free article] [PubMed] [Google Scholar]
- McQuibban G. A., Butler G. S., Gong J. H., Bendall L., Power C., Clark-Lewis I., Overall C. M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001 Sep 24;276(47):43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- McQuibban G. A., Gong J. H., Tam E. M., McCulloch C. A., Clark-Lewis I., Overall C. M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000 Aug 18;289(5482):1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- Mercer E. H., Hoyle G. W., Kapur R. P., Brinster R. L., Palmiter R. D. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991 Nov;7(5):703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- Mercuri F. A., Maciewicz R. A., Tart J., Last K., Fosang A. J. Mutations in the interglobular domain of aggrecan alter matrix metalloproteinase and aggrecanase cleavage patterns. Evidence that matrix metalloproteinase cleavage interferes with aggrecanase activity. J Biol Chem. 2000 Oct 20;275(42):33038–33045. doi: 10.1074/jbc.275.42.33038. [DOI] [PubMed] [Google Scholar]
- Mitchell P. G., Magna H. A., Reeves L. M., Lopresti-Morrow L. L., Yocum S. A., Rosner P. J., Geoghegan K. F., Hambor J. E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996 Feb 1;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhold L. A., Killar L., Zhao W., Sung M. L., Warner L., Kulik J., Turner J., Wu W., Billinghurst C., Meijers T. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001 Jan;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita I., Ghivizzani S. C., Galea-Lauri J., Bandara G., Georgescu H. I., Robbins P. D., Evans C. H. Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. 1996 May;39(5):820–828. doi: 10.1002/art.1780390515. [DOI] [PubMed] [Google Scholar]
- Omura T. H., Noguchi A., Johanns C. A., Jeffrey J. J., Partridge N. C. Identification of a specific receptor for interstitial collagenase on osteoblastic cells. J Biol Chem. 1994 Oct 7;269(40):24994–24998. [PubMed] [Google Scholar]
- Otterness I. G., Bliven M. L., Eskra J. D., te Koppele J. M., Stukenbrok H. A., Milici A. J. Cartilage damage after intraarticular exposure to collagenase 3. Osteoarthritis Cartilage. 2000 Sep;8(5):366–373. doi: 10.1053/joca.1999.0311. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Downs J. T., Lane C., Bliven M. L., Stukenbrok H., Scampoli D. N., Milici A. J., Mézes P. S. Detection of collagenase-induced damage of collagen by 9A4, a monoclonal C-terminal neoepitope antibody. Matrix Biol. 1999 Aug;18(4):331–341. doi: 10.1016/s0945-053x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Overall Christopher M., McQuibban G. Angus, Clark-Lewis Ian. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol Chem. 2002 Jul-Aug;383(7-8):1059–1066. doi: 10.1515/BC.2002.114. [DOI] [PubMed] [Google Scholar]
- Pap T., Shigeyama Y., Kuchen S., Fernihough J. K., Simmen B., Gay R. E., Billingham M., Gay S. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000 Jun;43(6):1226–1232. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Parks W. C., Shapiro S. D. Matrix metalloproteinases in lung biology. Respir Res. 2000 Dec 29;2(1):10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podworny N. V., Kandel R. A., Renlund R. C., Grynpas M. D. Partial chondroprotective effect of zoledronate in a rabbit model of inflammatory arthritis. J Rheumatol. 1999 Sep;26(9):1972–1982. [PubMed] [Google Scholar]
- Salminen H. J., Sämänen A-M K., Vankemmelbeke M. N., Auho P. K., Perälä M. P., Vuorio E. I. Differential expression patterns of matrix metalloproteinases and their inhibitors during development of osteoarthritis in a transgenic mouse model. Ann Rheum Dis. 2002 Jul;61(7):591–597. doi: 10.1136/ard.61.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk S. J., Boivin G. P., Duwel L. E., Ball W., Bove K., Trapnell B., Hirsch R. Anti-T cell receptor monoclonal antibody prolongs transgene expression following adenovirus-mediated in vivo gene transfer to mouse synovium. Hum Gene Ther. 1996 Mar 1;7(4):499–506. doi: 10.1089/hum.1996.7.4-499. [DOI] [PubMed] [Google Scholar]
- Schönbeck U., Mach F., Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998 Oct 1;161(7):3340–3346. [PubMed] [Google Scholar]
- Shlopov B. V., Smith G. N., Jr, Cole A. A., Hasty K. A. Differential patterns of response to doxycycline and transforming growth factor beta1 in the down-regulation of collagenases in osteoarthritic and normal human chondrocytes. Arthritis Rheum. 1999 Apr;42(4):719–727. doi: 10.1002/1529-0131(199904)42:4<719::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sandstedt B., Bruce K., Lindahl A., Jiménez M. G., Vega J. A., López-Otín C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997 May;76(5):717–728. [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. E. Update on synovitis. Curr Rheumatol Rep. 2001 Feb;3(1):53–63. doi: 10.1007/s11926-001-0051-0. [DOI] [PubMed] [Google Scholar]
- Tierney G. M., Griffin N. R., Stuart R. C., Kasem H., Lynch K. P., Lury J. T., Brown P. D., Millar A. W., Steele R. J., Parsons S. L. A pilot study of the safety and effects of the matrix metalloproteinase inhibitor marimastat in gastric cancer. Eur J Cancer. 1999 Apr;35(4):563–568. doi: 10.1016/s0959-8049(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Vu T. H., Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000 Sep 1;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- Whitelock J. M., Murdoch A. D., Iozzo R. V., Underwood P. A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996 Apr 26;271(17):10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992 May 11;20(9):2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., Nakamura H., Obata K., Yamada H., Hayakawa T., Fujikawa K., Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000 Jun;59(6):455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000 Jan 15;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]