Abstract
Objectives: To analyse immunological changes during treatment with a monoclonal anti-tumour necrosis factor α (TNFα) antibody, infliximab, in patients with rheumatoid arthritis (RA).
Methods: 25 patients with RA and 5 patients with other arthritides were studied during the first 6 weeks of treatment with infliximab. At the start of treatment and after 2 and 6 weeks, spontaneous expression of CCR3 and CCR5 on peripheral blood T cells and monocytes was studied by flow cytometry. The secretion and mRNA expression of interferon γ (IFNγ), interleukin (IL)4, IL5, and TNFα from phytohaemagglutinin (PHA) stimulated peripheral blood mononuclear cells was measured with an ELISA and RT-PCR. Plasma levels of C reactive protein, serum amyloid protein A, rheumatoid factor, and antibodies to filaggrin and citrullinated cyclic peptide were measured with an ELISA.
Results: The number of CD4 T cells and CD14 monocytes expressing CCR3 (p = 0.013, p = 0.009, respectively) and CD8 T cells expressing CCR5 (p = 0.040) as well as PHA stimulated secretion of IL4 and IFNγ (p<0.05) increased during treatment in patients with RA. 15 (60%) patients with RA achieved clinical response (at least ACR20) during the first 2 weeks. The number of T cells expressing CCR3 and CCR5 was higher before treatment in non-responders than in responders (p<0.05). The number of T cells increased in responders.
Conclusion: Increase in secretion of Th1 and Th2 cytokines together with induced expression of chemokine receptors on T cells and monocytes suggest restoration of peripheral cell mediated immunity and blockade of the accumulation of inflammatory cells in joints as response to treatment.
Full Text
The Full Text of this article is available as a PDF (176.0 KB).
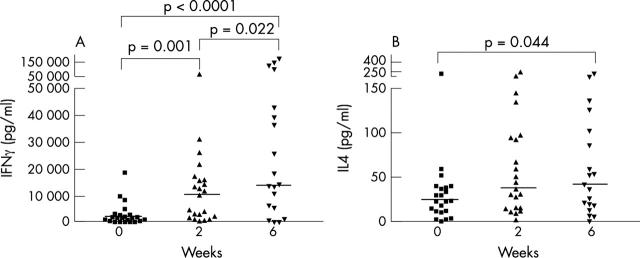
Figure 1 .
Effect of infliximab on the secretion of IFNγ (A) and IL4 (B), studied with an ELISA, from PHA stimulated PBMC in patients with RA. The median values are indicated with horizontal lines.
Figure 2 .
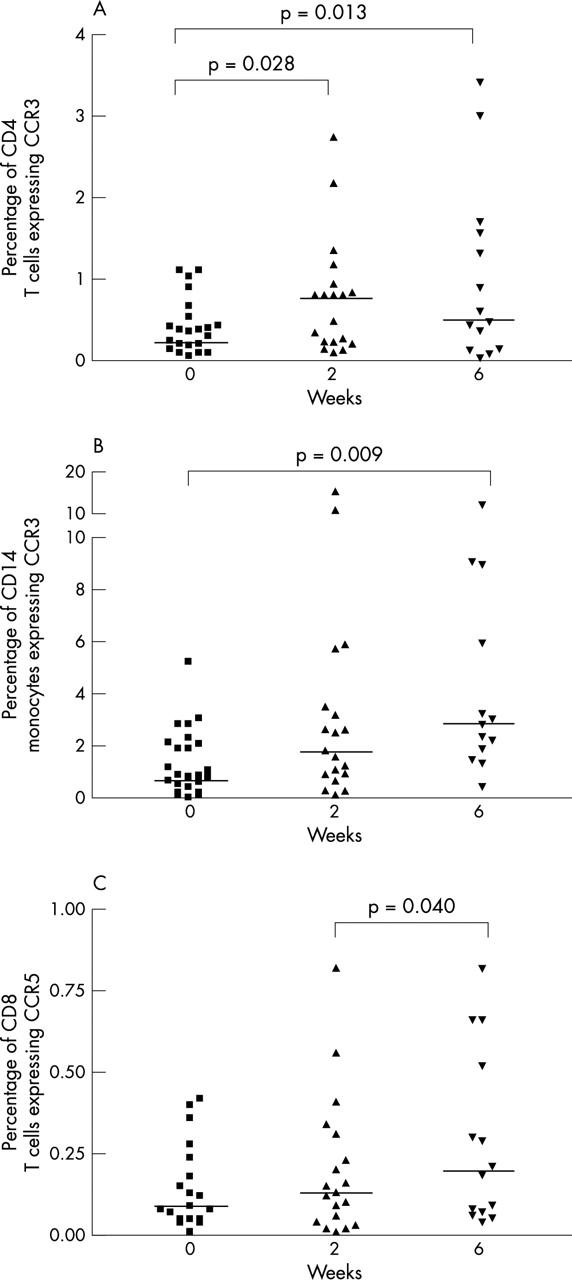
Effect of infliximab treatment on the percentage of (A) CD4 T cells expressing CCR3; (B) CD14 gated monocytes expressing CCR3; and (C) CD8 T cells expressing CCR5 collected from CD3 gate and studied with flow cytometry in patients with RA. The median values are indicated with horizontal lines.
Figure 3 .
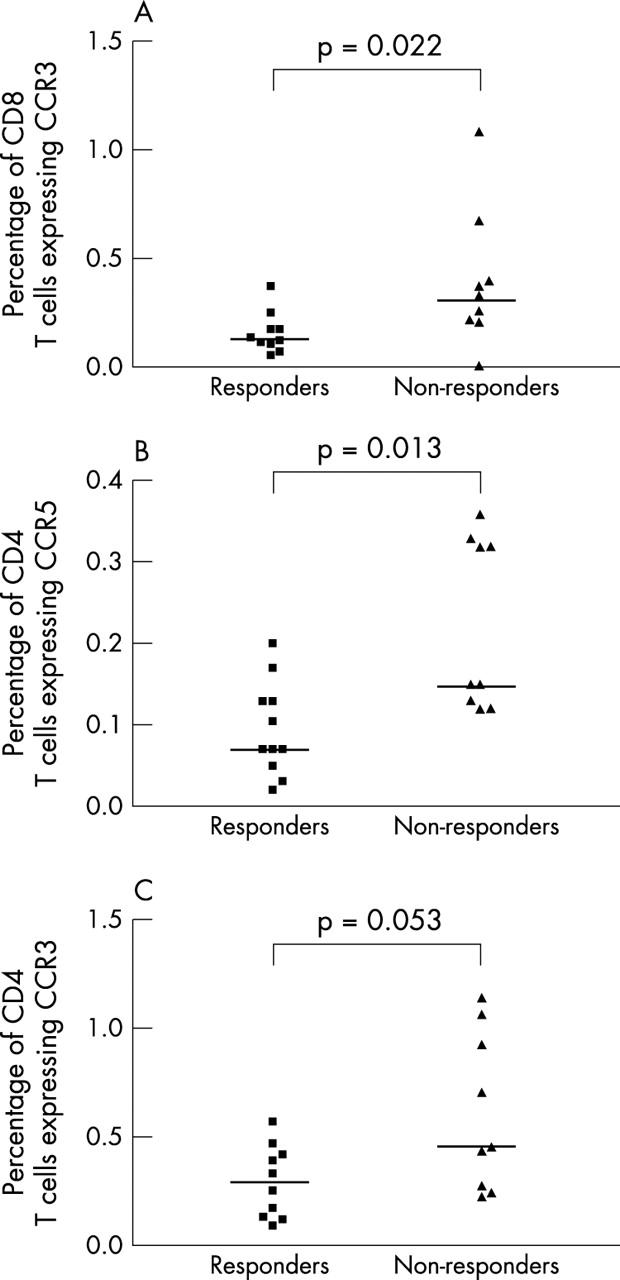
The percentage of CD8 (A) and CD4 (B, C) T cells expressing CCR3 (A, C) and CCR5 (B), studied with flow cytometry, before the start of treatment in patients with RA who responded and did not respond to the treatment. The median values are indicated with horizontal lines.
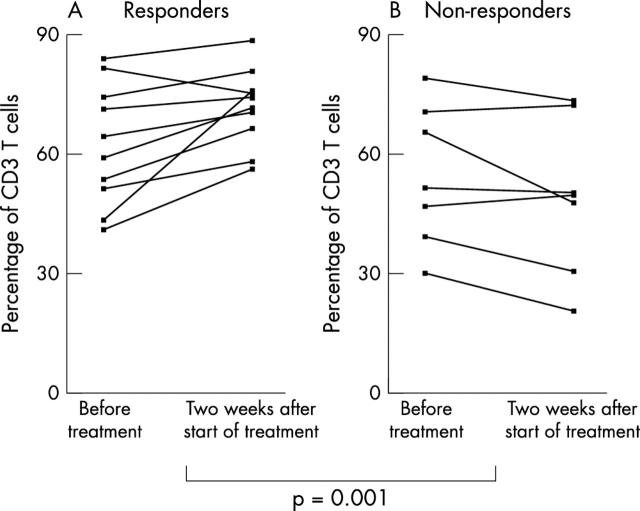
Figure 4 .
Change in the number of CD3 T cells (A, B) during infliximab treatment studied with flow cytometry in patients with RA responding (A) and not responding (B) to treatment. A comparison of the number of T cells between the groups showed a significant difference (p = 0.001).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeten D., Van Damme N., Van den Bosch F., Kruithof E., De Vos M., Mielants H., Veys E. M., De Keyser F. Impaired Th1 cytokine production in spondyloarthropathy is restored by anti-TNFalpha. Ann Rheum Dis. 2001 Aug;60(8):750–755. doi: 10.1136/ard.60.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L., Lampa J., Rogberg S., van Vollenhoven R., Klareskog L. Increased peripheral T cell reactivity to microbial antigens and collagen type II in rheumatoid arthritis after treatment with soluble TNFalpha receptors. Ann Rheum Dis. 2001 Feb;60(2):133–139. doi: 10.1136/ard.60.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P., Elliott M. J., Davis D., Potter A., Kalden J. R., Antoni C., Breedveld F. C., Smolen J. S., Eberl G., deWoody K. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999 Aug 1;163(3):1521–1528. [PubMed] [Google Scholar]
- Choy E. H., Panayi G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Cope A. P., Londei M., Chu N. R., Cohen S. B., Elliott M. J., Brennan F. M., Maini R. N., Feldmann M. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994 Aug;94(2):749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F., Paleolog E., Taylor P., Maini R. N. Anti-tumor necrosis factor alpha therapy of rheumatoid arthritis. Mechanism of action. Eur Cytokine Netw. 1997 Sep;8(3):297–300. [PubMed] [Google Scholar]
- Felson D. T., Anderson J. J., Boers M., Bombardier C., Furst D., Goldsmith C., Katz L. M., Lightfoot R., Jr, Paulus H., Strand V. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995 Jun;38(6):727–735. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung F., Scala G., Lenardo M. J. TNF-alpha-induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000 Jun 15;164(12):6180–6187. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- Lane B. R., Markovitz D. M., Woodford N. L., Rochford R., Strieter R. M., Coffey M. J. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999 Oct 1;163(7):3653–3661. [PubMed] [Google Scholar]
- Lorenz H. M., Antoni C., Valerius T., Repp R., Grünke M., Schwerdtner N., Nüsslein H., Woody J., Kalden J. R., Manger B. In vivo blockade of TNF-alpha by intravenous infusion of a chimeric monoclonal TNF-alpha antibody in patients with rheumatoid arthritis. Short term cellular and molecular effects. J Immunol. 1996 Feb 15;156(4):1646–1653. [PubMed] [Google Scholar]
- Maini R. N., Breedveld F. C., Kalden J. R., Smolen J. S., Davis D., Macfarlane J. D., Antoni C., Leeb B., Elliott M. J., Woody J. N. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998 Sep;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McKenna R. M., Wilkins J. A., Warrington R. J. Lymphokine production in rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1988 Nov;15(11):1639–1642. [PubMed] [Google Scholar]
- Paleolog E. M., Hunt M., Elliott M. J., Feldmann M., Maini R. N., Woody J. N. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor alpha antibody in rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1082–1091. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- Paleolog E. M., Young S., Stark A. C., McCloskey R. V., Feldmann M., Maini R. N. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum. 1998 Jul;41(7):1258–1265. doi: 10.1002/1529-0131(199807)41:7<1258::AID-ART17>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Palosuo T., Lukka M., Alenius H., Kalkkinen N., Aho K., Kurki P., Heikkilä R., Nykänen M., von Essen R. Purification of filaggrin from human epidermis and measurement of antifilaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998 Apr;115(4):294–302. doi: 10.1159/000069460. [DOI] [PubMed] [Google Scholar]
- Sadouk M., Vaquero C., de la Tour B., Amor B., Toubert A. Interferon-gamma mRNA expression upon in vitro T lymphocyte activation is decreased in rheumatoid arthritis patients. Clin Immunol Immunopathol. 1990 Jul;56(1):37–45. doi: 10.1016/0090-1229(90)90167-o. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A., Mackay C. R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998 Dec;19(12):568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Palermo B., Lenig D., Miettinen M., Matikainen S., Julkunen I., Forster R., Burgstahler R., Lipp M., Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999 May;29(5):1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sato K., Kawasaki H., Nagayama H., Enomoto M., Morimoto C., Tadokoro K., Juji T., Takahashi T. A. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol. 2000 Mar 1;164(5):2285–2295. doi: 10.4049/jimmunol.164.5.2285. [DOI] [PubMed] [Google Scholar]
- Schellekens G. A., Visser H., de Jong B. A., van den Hoogen F. H., Hazes J. M., Breedveld F. C., van Venrooij W. J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000 Jan;43(1):155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Seitz M., Napierski I., Augustin R., Hunstein W., Kirchner H. Reduced production of interferon alpha and interferon gamma in leukocyte cultures from patients with active rheumatoid arthritis. Scand J Rheumatol. 1987;16(4):257–262. doi: 10.3109/03009748709102926. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kremer J. M., Bankhurst A. D., Bulpitt K. J., Fleischmann R. M., Fox R. I., Jackson C. G., Lange M., Burge D. J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999 Jan 28;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]