Abstract
Background: Serum amyloid P component (SAP) and acute phase proteins like C-reactive protein contribute to the clearance of apoptotic cells. This response is diminished in systemic lupus erythematosus (SLE).
Objectives: To analyse SAP concentrations in SLE in relation to disease activity, and investigate whether SAP reacts like an acute phase protein.
Methods: SAP was measured in 40 patients with SLE during active and inactive disease and compared with healthy controls and patients with rheumatoid arthritis and Wegener's granulomatosis. Normal SAP values were determined in 120 healthy controls by ELISA. C reactive protein and serum amyloid A (SAA) were measured in all subjects and their levels related to SAP. SAP was also measured serially in 11 patients with breast cancer treated with recombinant human interleukin-6, and in 16 patients with sepsis.
Results: In SLE, SAP was unaltered compared with healthy controls and was not influenced by disease activity, in contrast to C reactive protein and SAA, which increased during active disease. SAP increased in Wegener's granulomatosis but not in rheumatoid arthritis. The rise in C reactive protein and SAA was most pronounced in Wegener's granulomatosis with active disease. SAP did not change significantly during an acute phase response. No correlation was found between SAP and C reactive protein or SAA, but there was a correlation between SAA and C reactive protein (r = 0.4989, p = 0.0492).
Conclusions: Patients with SLE have normal circulating SAP levels. In contrast to C reactive protein or SAA, SAP does not act as an acute phase protein.
Full Text
The Full Text of this article is available as a PDF (130.9 KB).
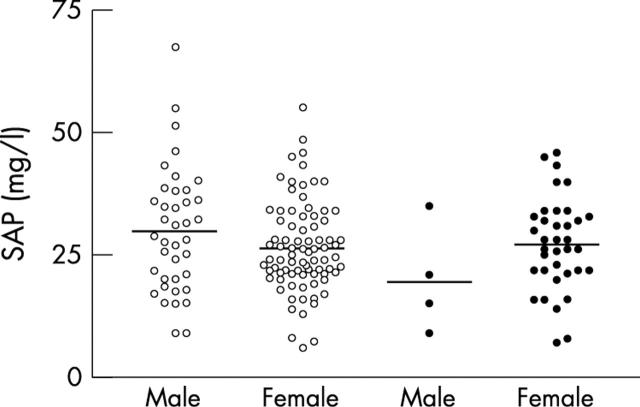
Figure 1.
Serum amyloid P component (SAP) in male (n = 39) and female (n = 81) healthy controls (empty circles) and in male (n = 4) and female (n = 36) SLE patients during inactive disease (filled circles). Horizontal bars indicate the mean.
Figure 2.
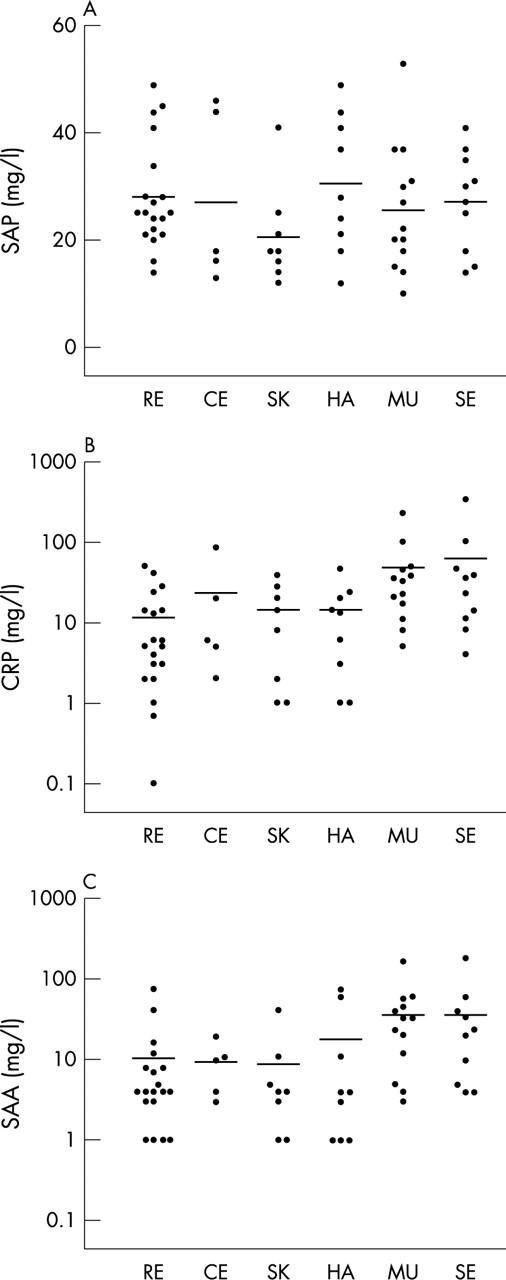
Serum amyloid P component (SAP) (A), C reactive protein (B), and serum amyloid A (SAA) (C) values in 40 patients with SLE during active disease. Disease manifestations were divided into organ manifestations based on the ACR criteria of SLE: RE, renal; CE, cerebral; SK, skin; HA, haematological; MU, musculoskeletal; SE, serositis. More than one organ system could be involved in an individual patient. Horizontal bars indicate the mean.
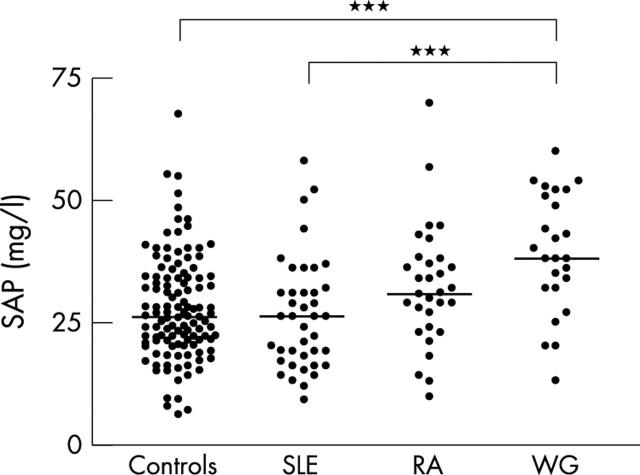
Figure 3.
Serum amyloid P component (SAP) in healthy controls (n = 120), SLE (n = 40), rheumatoid arthritis (n = 31), and Wegener's granulomatosis (n = 25). SAP in the patients was measured during inactive disease. Horizontal bars indicate the mean. ***p<0.001 by one way analysis of variance with Bonferroni's multiple comparison test.
Figure 4.

Serum amyloid P component (SAP) does not behave as an acute phase protein. (A) SAP, C reactive protein, and serum amyloid A (SAA) responses in a patient with breast cancer treated for seven days with recombinant interleukin 6 (rhIL-6) at a dose of 20 µg/kg/day. Even though a significant increase in C reactive protein (from18 to 395 mg/l on day 3) and an even more pronounced increase in SAA (from 11 to 770 mg/l on day 3) occurred, SAP levels hardly changed. (B) SAP levels of the same patient in more detail, showing a decline in SAP up to day 3, at the maximum of the acute phase response, and a slight increase thereafter.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bickerstaff M. C., Botto M., Hutchinson W. L., Herbert J., Tennent G. A., Bybee A., Mitchell D. A., Cook H. T., Butler P. J., Walport M. J. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999 Jun;5(6):694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- Bijl Marc, Horst Gerda, Bijzet Johan, Bootsma Hendrika, Limburg Pieter C., Kallenberg Cees G. M. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003 Jan;48(1):248–254. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Tennent G. A., Pepys M. B. Pentraxin-chromatin interactions: serum amyloid P component specifically displaces H1-type histones and solubilizes native long chromatin. J Exp Med. 1990 Jul 1;172(1):13–18. doi: 10.1084/jem.172.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Courtney P. A., Crockard A. D., Williamson K., Irvine A. E., Kennedy R. J., Bell A. L. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999 May;58(5):309–314. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familian A., Zwart B., Huisman H. G., Rensink I., Roem D., Hordijk P. L., Aarden L. A., Hack C. E. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001 Jul 15;167(2):647–654. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- Fazzini F., Peri G., Doni A., Dell'Antonio G., Dal Cin E., Bozzolo E., D'Auria F., Praderio L., Ciboddo G., Sabbadini M. G. PTX3 in small-vessel vasculitides: an independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001 Dec;44(12):2841–2850. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Franssen C. F., Stegeman C. A., Oost-Kort W. W., Kallenberg C. G., Limburg P. C., Tiebosch A., De Jong P. E., Tervaert J. W. Determinants of renal outcome in anti-myeloperoxidase-associated necrotizing crescentic glomerulonephritis. J Am Soc Nephrol. 1998 Oct;9(10):1915–1923. doi: 10.1681/ASN.V9101915. [DOI] [PubMed] [Google Scholar]
- Gershov D., Kim S., Brot N., Elkon K. B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000 Nov 6;192(9):1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R. Y., Fauci A. S., Bloch D. A., Michel B. A., Hunder G. G., Arend W. P., Calabrese L. H., Fries J. F., Lie J. T., Lightfoot R. W., Jr The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990 Aug;33(8):1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Zhou J. L., Song X., Elkon K. B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998 Jul 20;188(2):387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold C., Gresham H. D., Du Clos T. W. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001 Jan 15;166(2):1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- Patry Y. C., Trewick D. C., Gregoire M., Audrain M. A., Moreau A. M., Muller J. Y., Meflah K., Esnault V. L. Rats injected with syngenic rat apoptotic neutrophils develop antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2001 Aug;12(8):1764–1768. doi: 10.1681/ASN.V1281764. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M., Gomer K., Davies A. J., Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979 Mar 15;278(5701):259–261. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Booth S. E., Tennent G. A., Butler P. J., Williams D. G. Binding of pentraxins to different nuclear structures: C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol. 1994 Jul;97(1):152–157. doi: 10.1111/j.1365-2249.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Perniok A., Wedekind F., Herrmann M., Specker C., Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7(2):113–118. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- Posthumus Marcel D., Limburg Pieter C., Westra Johanna, van Leeuwen Miek A., van Rijswijk Martin H. Serum matrix metalloproteinase 3 levels during treatment with sulfasalazine or combination of methotrexate and sulfasalazine in patients with early rheumatoid arthritis. J Rheumatol. 2002 May;29(5):883–889. [PubMed] [Google Scholar]
- Rovere P., Peri G., Fazzini F., Bottazzi B., Doni A., Bondanza A., Zimmermann V. S., Garlanda C., Fascio U., Sabbadini M. G. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000 Dec 15;96(13):4300–4306. [PubMed] [Google Scholar]
- Sen J. W., Recke C., Rahbek L., Skogstrand K., Heegaard N. H. H. Structural, quantitative and functional comparison of amyloid P component in sera from patients with systemic lupus erythematosus and healthy donors. Scand J Immunol. 2002 Dec;56(6):645–651. doi: 10.1046/j.1365-3083.2002.01178.x. [DOI] [PubMed] [Google Scholar]
- Skinner M., Vaitukaitis J. L., Cohen A. S., Benson M. D. Serum amyloid P-component levels in amyloidosis, connective tissue diseases, infection, and malignancy as compared to normal serum. J Lab Clin Med. 1979 Oct;94(4):633–638. [PubMed] [Google Scholar]
- Spronk P. E., ter Borg E. J., Kallenberg C. G. Patients with systemic lupus erythematosus and Jaccoud's arthropathy: a clinical subset with an increased C reactive protein response? Ann Rheum Dis. 1992 Mar;51(3):358–361. doi: 10.1136/ard.51.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk P. E., ter Borg E. J., Limburg P. C., Kallenberg C. G. Plasma concentration of IL-6 in systemic lupus erythematosus; an indicator of disease activity? Clin Exp Immunol. 1992 Oct;90(1):106–110. doi: 10.1111/j.1365-2249.1992.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan A. F., Johnson P. M. Protein SAP (serum amyloid P-component) in Waldenström's macroglobulinaemia, multiple myeloma and rheumatic diseases. J Clin Lab Immunol. 1982 Sep;8(3):153–156. [PubMed] [Google Scholar]
- Sørensen I. J., Holm Nielsen E., Schrøder L., Voss A., Horváth L., Svehag S. E. Complexes of serum amyloid P component and DNA in serum from healthy individuals and systemic lupus erythematosus patients. J Clin Immunol. 2000 Nov;20(6):408–415. doi: 10.1023/a:1026478914129. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Huitema M. G., Hené R. J., Sluiter W. J., The T. H., van der Hem G. K., Kallenberg C. G. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990 Sep 22;336(8717):709–711. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- de Jong K. P., van Gameren M. M., Bijzet J., Limburg P. C., Sluiter W. J., Slooff M. J., de Vries E. G. Recombinant human interleukin-6 induces hepatocyte growth factor production in cancer patients. Scand J Gastroenterol. 2001 Jun;36(6):636–640. doi: 10.1080/003655201750163132. [DOI] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E. J., Limburg P. C., Kallenberg C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990 May;33(5):634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Limburg P. C., van Rijswijk M. H., Kallenberg C. G. C-reactive protein levels during disease exacerbations and infections in systemic lupus erythematosus: a prospective longitudinal study. J Rheumatol. 1990 Dec;17(12):1642–1648. [PubMed] [Google Scholar]