Abstract
Background: Paracetamol is a recommended symptomatic treatment of osteoarthritis (OA), but in clinical trials sample sizes have been relatively small and variable daily doses of paracetamol have been used.
Objectives: To determine the therapeutic efficacy of paracetamol in OA of the knee and identify predictive factors of clinical response to treatment.
Methods: A double blind, parallel group, placebo controlled trial of analgesic efficacy and safety of paracetamol versus placebo including 779 patients with OA of the knee. Patients were randomly assigned to receive paracetamol 4 g/day (n = 405) or placebo (n = 374) for 6 weeks. Symptomatic OA of the knee was required at inclusion with global pain intensity of the knee during physical activities for the past 24 hours of ⩾30 mm on a 100 mm visual analogue scale. The primary end point was a 30% decrease of global pain intensity of the knee. Intention to treat analyses were performed.
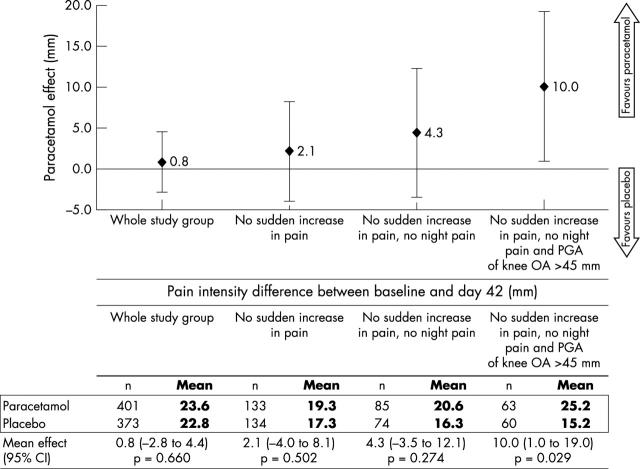
Results: The percentage of responders did not differ significantly between groups: 52.6% and 51.9% in paracetamol and placebo groups, respectively (p = 0.840). In a subgroup of patients with chronic mechanical knee pain without signs of inflammation (n = 123), the mean change in pain intensity from baseline was 25.2 mm v 15.2 mm, in the paracetamol (n = 63) and placebo (n = 60) groups, respectively—mean difference 10.0 mm; 95% CI 1.0 to 19.0; p = 0.0294. No serious adverse events were attributable to treatment.
Conclusion: A statistically significant symptomatic effect of oral paracetamol 4 g/day over placebo was not found, suggesting that paracetamol use in symptomatic OA of the knee should be further explored. The tolerability and safety of paracetamol, at the recommended maximum dose of 4 g/day, was confirmed over 6 weeks.
Full Text
The Full Text of this article is available as a PDF (216.8 KB).
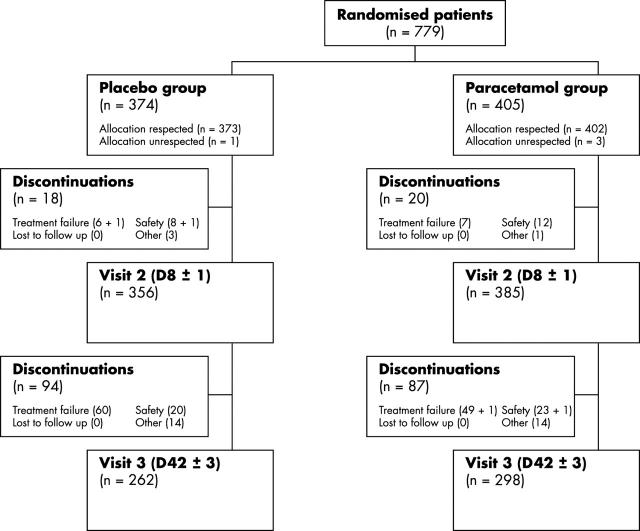
Figure 1.
Patients' disposition.
Figure 2.
Treatment effect (differences in changes in pain intensity between the two study groups) in different selected subgroups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy N., Buchanan W. W., Goldsmith C. H., Campbell J., Stitt L. W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988 Dec;15(12):1833–1840. [PubMed] [Google Scholar]
- Bensen W. G., Fiechtner J. J., McMillen J. I., Zhao W. W., Yu S. S., Woods E. M., Hubbard R. C., Isakson P. C., Verburg K. M., Geis G. S. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc. 1999 Nov;74(11):1095–1105. doi: 10.4065/74.11.1095. [DOI] [PubMed] [Google Scholar]
- Bradley J. D., Brandt K. D., Katz B. P., Kalasinski L. A., Ryan S. I. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal antiinflammatory drug or pure analgesic. J Rheumatol. 1992 Dec;19(12):1950–1954. [PubMed] [Google Scholar]
- Case John P., Baliunas Algis J., Block Joel A. Lack of efficacy of acetaminophen in treating symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled comparison trial with diclofenac sodium. Arch Intern Med. 2003 Jan 27;163(2):169–178. doi: 10.1001/archinte.163.2.169. [DOI] [PubMed] [Google Scholar]
- Dougados M., Leclaire P., van der Heijde D., Bloch D. A., Bellamy N., Altman R. D. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000 Nov;8(6):395–403. doi: 10.1053/joca.2000.0361. [DOI] [PubMed] [Google Scholar]
- Dougados M., Leclaire P., van der Heijde D., Bloch D. A., Bellamy N., Altman R. D. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000 Nov;8(6):395–403. doi: 10.1053/joca.2000.0361. [DOI] [PubMed] [Google Scholar]
- Eccles M., Freemantle N., Mason J. North of England evidence based guideline development project: summary guideline for non-steroidal anti-inflammatory drugs versus basic analgesia in treating the pain of degenerative arthritis. The North of England Non-Steroidal Anti-Inflammatory Drug Guideline Development Group. BMJ. 1998 Aug 22;317(7157):526–530. doi: 10.1136/bmj.317.7157.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E. W., Schnitzer T. J., McIlwain H., Levy R., Wolfe F., Weisman M., Zeng Q., Morrison B., Bolognese J., Seidenberg B. Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. Rofecoxib Osteoarthritis Pilot Study Group. J Rheumatol. 1999 Nov;26(11):2438–2447. [PubMed] [Google Scholar]
- Fored C. M., Ejerblad E., Lindblad P., Fryzek J. P., Dickman P. W., Signorello L. B., Lipworth L., Elinder C. G., Blot W. J., McLaughlin J. K. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001 Dec 20;345(25):1801–1808. doi: 10.1056/NEJMoa010323. [DOI] [PubMed] [Google Scholar]
- Geba Gregory P., Weaver Arthur L., Polis Adam B., Dixon Mary E., Schnitzer Thomas J., Vioxx, Acetaminophen, Celecoxib Trial (VACT) Group Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA. 2002 Jan 2;287(1):64–71. doi: 10.1001/jama.287.1.64. [DOI] [PubMed] [Google Scholar]
- Graham Garry G., Graham Robert I., Day Richard O. Comparative analgesia, cardiovascular and renal effects of celecoxib, rofecoxib and acetaminophen (paracetamol). Curr Pharm Des. 2002;8(12):1063–1075. doi: 10.2174/1381612023394917. [DOI] [PubMed] [Google Scholar]
- Hochberg M. C., Altman R. D., Brandt K. D., Clark B. M., Dieppe P. A., Griffin M. R., Moskowitz R. W., Schnitzer T. J. Guidelines for the medical management of osteoarthritis. Part I. Osteoarthritis of the hip. American College of Rheumatology. Arthritis Rheum. 1995 Nov;38(11):1535–1540. doi: 10.1002/art.1780381103. [DOI] [PubMed] [Google Scholar]
- Jordan K. M., Arden N. K., Doherty M., Bannwarth B., Bijlsma J. W. J., Dieppe P., Gunther K., Hauselmann H., Herrero-Beaumont G., Kaklamanis P. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003 Dec;62(12):1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLGREN J. H., LAWRENCE J. S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuay H. J., Poppleton P., Carroll D., Summerfield R. J., Bullingham R. E., Moore R. A. Ketorolac and acetaminophen for orthopedic postoperative pain. Clin Pharmacol Ther. 1986 Jan;39(1):89–93. doi: 10.1038/clpt.1986.15. [DOI] [PubMed] [Google Scholar]
- Pendleton A., Arden N., Dougados M., Doherty M., Bannwarth B., Bijlsma J. W., Cluzeau F., Cooper C., Dieppe P. A., Günther K. P. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000 Dec;59(12):936–944. doi: 10.1136/ard.59.12.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Koch G. G., Sokka T., Lefkowith J., Wolfe F., Jordan J. M., Luta G., Callahan L. F., Wang X., Schwartz T. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2001 Jul;44(7):1587–1598. doi: 10.1002/1529-0131(200107)44:7<1587::AID-ART282>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pincus T., Koch G., Lei H., Mangal B., Sokka T., Moskowitz R., Wolfe F., Gibofsky A., Simon L., Zlotnick S. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis. 2004 Apr 13;63(8):931–939. doi: 10.1136/ard.2003.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott L. F. Therapeutic misadventure with paracetamol: fact or fiction? Am J Ther. 2000 Mar;7(2):99–114. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]
- Schug S. A., Sidebotham D. A., McGuinnety M., Thomas J., Fox L. Acetaminophen as an adjunct to morphine by patient-controlled analgesia in the management of acute postoperative pain. Anesth Analg. 1998 Aug;87(2):368–372. doi: 10.1097/00000539-199808000-00024. [DOI] [PubMed] [Google Scholar]
- Seymour R. A., Kelly P. J., Hawkesford J. E. The efficacy of ketoprofen and paracetamol (acetaminophen) in postoperative pain after third molar surgery. Br J Clin Pharmacol. 1996 Jun;41(6):581–585. doi: 10.1046/j.1365-2125.1996.34015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: a view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am J Ther. 2000 Mar;7(2):115–121. doi: 10.1097/00045391-200007020-00008. [DOI] [PubMed] [Google Scholar]
- Skoglund L. A., Skjelbred P., Fyllingen G. Analgesic efficacy of acetaminophen 1000 mg, acetaminophen 2000 mg, and the combination of acetaminophen 1000 mg and codeine phosphate 60 mg versus placebo in acute postoperative pain. Pharmacotherapy. 1991;11(5):364–369. [PubMed] [Google Scholar]
- Skovlund E., Fyllingen G., Landre H., Nesheim B. I. Comparison of postpartum pain treatments using a sequential trial design: II. Naproxen versus paracetamol. Eur J Clin Pharmacol. 1991;40(6):539–542. doi: 10.1007/BF00279965. [DOI] [PubMed] [Google Scholar]
- Steiner T. J., Lange R. Ketoprofen (25 mg) in the symptomatic treatment of episodic tension-type headache: double-blind placebo-controlled comparison with acetaminophen (1000 mg). Cephalalgia. 1998 Jan;18(1):38–43. doi: 10.1046/j.1468-2982.1998.1801038.x. [DOI] [PubMed] [Google Scholar]
- Williams H. J., Ward J. R., Egger M. J., Neuner R., Brooks R. H., Clegg D. O., Field E. H., Skosey J. L., Alarcón G. S., Willkens R. F. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum. 1993 Sep;36(9):1196–1206. doi: 10.1002/art.1780360904. [DOI] [PubMed] [Google Scholar]