Abstract
Fungi play crucial roles in the biogeochemistry of terrestrial ecosystems, most notably as saprophytes decomposing organic matter and as mycorrhizal fungi enhancing plant nutrient uptake. However, a recurrent problem in fungal ecology is to establish the trophic status of species in the field. Our interpretations and conclusions are too often based on extrapolations from laboratory microcosm experiments or on anecdotal field evidence. Here, we used natural variations in stable carbon isotope ratios (δ13C) as an approach to distinguish between fungal decomposers and symbiotic mycorrhizal fungal species in the rich sporocarp flora (our sample contains 135 species) of temperate forests. We also demonstrated that host-specific mycorrhizal fungi that receive C from overstorey or understorey tree species differ in their δ13C. The many promiscuous mycorrhizal fungi, associated with and connecting several tree hosts, were calculated to receive 57–100% of their C from overstorey trees. Thus, overstorey trees also support, partly or wholly, the nutrient-absorbing mycelia of their alleged competitors, the understorey trees.
In terrestrial ecosystems, fungi are integral components of most biogeochemical cycles. Decomposer fungi degrade organic macromolecules whereas mycorrhizal fungi, symbionts of plant roots, actively forage for plant nutrients (1, 2). In the complex settings of forest ecosystems, even in those with few plant species, there is often a diverse flora of sporocarps of fungal decomposers and mycorrhizal fungi (3–5). However, the trophic status of many fungal species remains unclear: that is, whether they obtain their C by decomposing dead organic material or from living plants by forming mycorrhizal root symbioses (6). The small size and cryptic growth form of most fungal mycelia effectively restricts in situ observations. Furthermore, the connection between mycorrhizal fungi and their host trees cannot be easily established because many fungi are promiscuous and can receive C from several species of trees (2, 7, 8). Such fungi form hyphal links transferring C and nutrients between plants and can thus influence plant competition (2, 7–10). Recently, it was suggested, after feeding one seedling with 14CO2 and one with 13CO2, that there was a net transfer of C from one seedling to another via mycorrhizal connections (8). However, this type of tracer approach is highly elaborate and cannot easily be used to study a greater complexity: that is, flows of C to many fungal species simultaneously.
Fungal decomposers break down complex C sources like cellulose, hemicellulose, and lignin whereas mycorrhizal fungi receive simpler carbohydrates directly via the plant phloem (1, 2). These C sources may differ significantly in their natural abundance of the heavy stable carbon isotope 13C (11, 12). Accordingly, we speculated (i) that analysis of 13C abundance could differentiate between saprophytic and mycorrhizal fungi.
We also hypothesized (ii) that, because overstorey trees generally have a higher natural abundance of 13C than understorey trees (13), their associated ectomycorrhizal fungi should display similar differences. The higher 13C abundance of overstorey trees is the result of less pronounced discrimination against 13C in CO2 during photosynthesis because of higher illumination (and, therefore, higher rates of photosynthesis) and also because of more exposure to drought stress in overstorey trees (13–15). Short plants in a closed forest may obtain particularly low 13C abundance through assimilation of 13C-depleted CO2 derived directly from soil respiration (13).
We also hypothesized (iii) that most of the mycorrhizal fungi ought to receive their C from overstorey rather than from understorey trees because rates of photosynthesis are higher in overstorey trees. To test the three hypotheses (i–iii), we visited two two-storied, successional, temperate forests in Sweden and sampled trees and a large number of fungi to analyze their 13C abundance.
METHODS
The study was conducted at Åheden in the Svartberget Research Forest, 60 km northwest of Umeå, northern Sweden (64°14′N, 19°46′E, 175 m above sea level) and at Stadsskogen, an urban forest in Uppsala, central Sweden (59°52′N, 17°13′E, 35 m above sea level). Both forests had 150-year-old Scots pine (Pinus sylvestris L.) in the overstorey, but with Norway spruce (Picea abies [L.] Karst.) approaching the role of codominant. At Åheden, the dominant Scots pine were 25 m tall, and birch (Betula pendula Roth) was the only understorey species. At Stadsskogen, the dominant Scots pine sometimes reached >30 m, and understorey deciduous tree species included aspen (Populus tremula L.), birch (B. pendula), willow (Salix caprea L.), and alder (Alnus incana [L.] Moench).
At each site, leaves and current needles were sampled (>10 g dry weight/sample) from branches on the south side, close to the top of the tree, from up to 10 specimens per tree species. Between one and eight fruit bodies from each fungal species found at each site were sampled (Table 1). Sampling was conducted on an area of 1 hectare at Åheden in August 1997 and at 5 plots with an area of 0.25 hectare each at Stadsskogen in September 1997. Specificity between tree hosts and fungal symbionts was inferred from the literature relevant to the region (16, 17). Because associations between fungi and potential hosts are based primarily on repeated observations of fruit bodies and not on establishing direct contacts between the two symbionts, the question of specificity must be treated with some caution. In this study, fungal species were only regarded as specific to a single host genus when this was clearly indicated in the literature.
Table 1.
Natural 13C abundance (δ13C) of fungal fruit bodies sampled in mixed temperate forests at two sites, Åheden and Stadsskogen, in Sweden
| Host | n | δ13C value | SD | |
|---|---|---|---|---|
| Åheden site | ||||
| Mycorrhizal species | ||||
| Boletales | ||||
| Boletaceae | ||||
| Leccinum molle (Bon) Bon | Betula | 1 | −27.6 | |
| Leccinum scabrum (Bull. Ex Fr.) S.F. Gray | Betula | 4 | −27.5 | 0.4 |
| Leccinum variicolor Watl. | Betula | 4 | −27.6 | 0.8 |
| Leccinum versipelle (Fr.) Snell | Betula | 4 | −27.5 | 0.6 |
| Suillus variegatus (Swartz ex Fr.) O. Kuntze | Pinus | 6 | −25.0 | 0.4 |
| Gomphidiaceae | ||||
| Chroogomphus rutilus (Schff. ex Fr.) O.K. Miller | Pinus | 4 | −26.0 | 0.6 |
| Tricholomataceae | ||||
| Tricholoma flavovirens (Pers. ex Fr.) | Promiscuous | 4 | −25.4 | 0.8 |
| Tricholoma virgatum (Fr.:Fr.) Kumm. | Promiscuous | 1 | −25.2 | |
| Laccaria bicolor (Maire) Orton | Promiscuous | 2 | −25.3 | 0.6 |
| Amanitaceae | ||||
| Amanita muscaria (L.) Hook | Promiscuous | 5 | −26.6 | 1.0 |
| Cortinariaceae | ||||
| Cortinarius armeniacus (Schaeff.:Fr.) Fr. | Conifers | 5 | −24.5 | 0.6 |
| Cortinarius armillatus (Fr.) Fr. | Betula | 5 | −26.9 | 1.3 |
| Cortinarius bataillei (Moser) Hiøland | Conifers | 1 | −25.3 | |
| Cortinarius biformis Fr. | Conifers | 4 | −25.2 | 0.6 |
| Cortinarius camphoratus (Fr.:Fr.) Fr. | Conifers | 1 | −25.6 | |
| Cortinarius evernuis (Fr.:Fr.) Fr. | Picea | 1 | −24.8 | |
| Cortinarius hemitrichus (Pers.:Fr.) Fr. | Betula | 1 | −27.8 | |
| Cortinarius laniger Fr. | Picea | 6 | −26.4 | 1.7 |
| Cortinarius malachius Fr. | Conifers | 2 | −24.8 | 0.2 |
| Cortinarius pholideus (Fr.:Fr.) Fr. | Betula | 6 | −27.6 | 1.0 |
| Cortinarius scaurus (Fr.:Fr.) Fr. | Promiscuous | 1 | −25.2 | |
| Cortinarius semisanguineus (Fr.) Gill. | Conifers | 3 | −24.9 | 0.7 |
| Cortinarius strobilaceus Mos. | Conifers | 1 | −27.0 | |
| Cortinarius traganus Fr. | (Pinus) | 2 | −25.2 | 0.5 |
| Rozites caperata (Pers. ex Fr.) Karst. | Promiscuous | 4 | −25.8 | 0.4 |
| Russulales | ||||
| Russulaceae | ||||
| Lactarius rufus (Scop.) Fr. | Promiscuous | 6 | −24.9 | 0.7 |
| Russula paludosa Britz. | Conifers | 1 | −25.0 | |
| Aphyllophorales | ||||
| Thelphoraceae | ||||
| Phelloden tomentosa (L.:Fr.) Baker | Promiscuous | 1 | −24.1 | |
| Saprophytic species | ||||
| Boletales | ||||
| Boletaceae | ||||
| Chalciporus piperatus (Bull. ex Fr.) Bat. | na | 1 | −22.3 | |
| Tricholomataceae | ||||
| Armillaria borealis Marx. & K. Korh. | na | 2 | −23.8 | 0.1 |
| Strophariaceae | ||||
| Stropharia hornemanii (Fr.:Fr.) Lund. | na | 1 | −23.1 | |
| Cortinariaceae | ||||
| Gymnopilus penetrans (Fr.) Murr. | 1 | −22.4 | ||
| Aphyllophorales | ||||
| Polyporaceae | ||||
| Trametes hirsuta (Wulfen:Fr.) Pil. | na | 1 | −24.0 | |
| Stadsskogen site | ||||
| Mycorrhizal species | ||||
| Boletales | ||||
| Boletaceae | ||||
| Boletus edulis Bull. ex Fr. | Promiscuous | 5 | −24.6 | 0.5 |
| Boletus pinophilus Pil. & Dermek | Pinus | 1 | −24.7 | |
| Leccinum aurantiacum (Bull. ex St. Am.) S.F. Gray | Populus | 3 | −27.3 | 0.9 |
| Leccinum holopus (Rostk.) Watl. | Betula | 1 | −29.2 | |
| Leccinum scabrum (Bull. ex Fr.) S.F. Gray | Betula | 6 | −26.4 | 1.9 |
| Leccinum variicolor Watl. | Betula | 1 | −26.1 | |
| Leccinum versipelle (Fr.) Snell | Betula | 2 | −27.1 | 1.1 |
| Leccinum vulpinum Watl. | Pinus | 5 | −25.0 | 0.7 |
| Suillus bovinus (L.) Kuntze | Pinus | 6 | −25.2 | 0.5 |
| Suillus luteus (L. ex Fr.) S.F. Gray | Pinus | 6 | −24.6 | 0.4 |
| Suillus variegatus (Swartz ex Fr.) O. Kuntze | Pinus | 7 | −24.3 | 0.9 |
| Tylopilus felleus (Bull.:Fr.) Karst. | Promiscuous | 5 | −25.0 | 0.5 |
| Xerocomus badius (Fr.:Fr.) Gilb. | Promiscuous | 4 | −25.3 | 0.8 |
| Xerocomus lanatus (Rostk.) Gilb. | Promiscuous | 1 | −25.9 | |
| Gomphidiaceae | ||||
| Chroogomphus rutilus (Schff. ex Fr.) O.K. Miller | Pinus | 6 | −25.3 | 0.7 |
| Gomphidius glutinosus (Schff.) Fr. | Picea | 3 | −26.4 | 0.6 |
| Gomphidius roseus L.:Fr. | Pinus | 5 | −24.9 | 0.7 |
| Paxillaceae | ||||
| Paxillus involutus (Batsch) Fr. | Promiscuous | 3 | −26.6 | 0.4 |
| Rhizopogonaceae | ||||
| Rhizopogon obtextus (Spreng) S. Rauschert | Pinus | 2 | −25.0 | 0.2 |
| Agaricales | ||||
| Hygrophoraceae | ||||
| Hygrophorus agathosmus (Fr.) Fr. | Picea | 1 | −28.1 | |
| Hygrophorus camarophyllus (Alb. & Schw.:Fr.), Dumée et al. | Conifers | 5 | −24.6 | 1.4 |
| Hygrophorus olivaceoalbus (Fr. ex Fr.) Fr. | Picea | 5 | −26.0 | 1.1 |
| Tricholomataceae | ||||
| Laccaria laccata (Scop. ex Fr.) Bk. & Br. | Promiscuous | 3 | −25.3 | 1.0 |
| Tricholoma flavovirens (Pers. ex Fr.) | Promiscuous | 2 | −26.1 | 0.8 |
| Tricholoma fracticum (Britz.) Kreisel | Pinus | 1 | −24.3 | |
| Tricholoma fucatum (Fr.) Kummer | Conifers | 2 | −25.8 | 0.6 |
| Tricholoma fulvum (DC:Fr.) Sacc. | Betula | 5 | −26.6 | 0.6 |
| Tricholoma vaccinum (Pers.:Fr) Kumm. | Picea | 1 | −26.9 | |
| Tricholoma virgatum (Fr.:Fr.) Kumm. | Promiscuous | 2 | −24.3 | 0.2 |
| Amanitaceae | ||||
| Amanita fulva (Sch:Fr) Fr. | (Betula) | 6 | −26.3 | 1.1 |
| Amanita muscaria (L.) Hook | Promiscuous | 4 | −26.3 | 0.7 |
| Amanita porphyria (Alb & Schw:Fr) Mlady | Conifers | 8 | −24.8 | 0.7 |
| Amanita rubescens (Pers:Fr.) SF Gray | Promiscuous | 5 | −25.3 | 0.5 |
| Amanita virosa (Kamarck) Bertillon | Promiscuous | 7 | −25.1 | 0.9 |
| Cortinariaceae | ||||
| Cortinarius albo-violaceus (Pers:Fr.) Fr. | Betula | 1 | −28.4 | |
| Cortinarius armeniacus (Schaeff.: Fr.) Fr. | Conifers | 1 | −25.1 | |
| Cortinarius armillatus (Fr.) Fr. | Betula | 3 | −27.5 | 0.9 |
| Cortinarius bolaris (Pers:Fr.) Fr. | (Betula) | 3 | −27.0 | 0.8 |
| Cortinarius brunneus Fr. | (Conifers) | 2 | −26.1 | 0.2 |
| Cortinarius camphoratus (Fr.:Fr.) Fr. | Conifers | 1 | −23.7 | |
| Cortinarius crocea (Schff.) Big. & Guill. | Promiscuous | 1 | −29.2 | |
| Cortinarius gentilis (Fr.) Fr. | (Picea) | 4 | −25.6 | 0.6 |
| Cortinarius laniger Fr. | (Picea) | 2 | −25.9 | 1.5 |
| Cortinarius limonius (Fr. ex Fr.) Fr. | Conifers | 2 | −25.9 | 2.6 |
| Cortinarius malachius Fr. | Conifers | 3 | −25.5 | 1.4 |
| Cortinarius muscigenus Peck | Picea | 3 | −26.1 | 0.8 |
| Cortinarius paleaceus (Weinm.) Fr. | Conifers | 7 | −25.7 | 1.3 |
| Cortinarius pholideus (Fr.:Fr.) Fr. | Betula | 1 | −26.3 | |
| Cortinarius semisanguineus (Fr.) Gill. | Conifers | 7 | −25.2 | 0.3 |
| Cortinarius speciosissimus Kühner & Rom. | Conifers | 1 | −25.8 | |
| Cortinarius stillatitius Fr. | Conifers | 3 | −24.2 | 1.1 |
| Cortinarius strobilaceus Mos. | Conifers | 3 | −25.5 | 0.8 |
| Cortinarius traganus Fr. | (Pinus) | 2 | −25.6 | 1.4 |
| Cortinarius uliginosus Berk. | Salix | 3 | −28.0 | 1.3 |
| Cortinarius vibratilis (Fr.:Fr.) Fr. | Conifers | 7 | −25.1 | 1.1 |
| Hebeloma crustuliniforme (Bull:Fr.) Quél. | Promiscuous | 4 | −27.1 | 2.1 |
| Hebeloma mesophaeum (Pers) Quél. | Promiscuous | 2 | −26.8 | 0.5 |
| Inocybe acuta Boud. | Promiscuous | 1 | −26.2 | |
| Inocybe cincinnata (Fr.) Quél. | Promiscuous | 1 | −26.5 | |
| Inocybe friesii Heim. | Conifers | 1 | −27.0 | |
| Inocybe geophylla (Sow. ex Fr.) Kummer | Promiscuous | 3 | −25.0 | 0.5 |
| Inocybe pseudodestricta Stangl. & Veselsky | Promiscuous | 1 | −26.5 | |
| Inocybe tigrina Heim | Conifers | 1 | −26.9 | |
| Rozites caperata (Pers. ex Fr.) Karst. | Promiscuous | 5 | −24.7 | 0.7 |
| Russulales | ||||
| Russulaceae | ||||
| Lactarius badiosanguinea Kuehn. & Rom. | Picea | 2 | −26.2 | 0.0 |
| Lactarius camphoratus (Bull.) ex Fr. | Promiscuous | 5 | −26.8 | 0.8 |
| Lactarius deliciosus Fr. | Pinus | 6 | −24.9 | 0.6 |
| Lactarius deterrimus Groeger | Picea | 8 | −26.6 | 0.8 |
| Lactarius fuliginosus Fr. | Betula | 2 | −24.9 | 0.4 |
| Lactarius glyciosmus Fr. | Betula | 4 | −27.0 | 0.5 |
| Lactarius helvus Fr. | Promiscuous | 4 | −23.8 | 0.4 |
| Lactarius mitissimus Fr. | Promiscuous | 1 | −25.1 | |
| Lactarius musteus Fr. | Pinus | 1 | −24.9 | |
| Lactarius necator (Bull. em Pers. ex Fr.) Karst. | Promiscuous | 5 | −26.0 | 1.1 |
| Lactarius obscuratus (Lasch) Fr. | Alnus | 3 | −27.1 | 0.6 |
| Lactarius repraesentaneus Britz. | Promiscuous | 1 | −26.5 | |
| Lactarius rufus (Scop.) Fr. | Promiscuous | 3 | −25.3 | 0.5 |
| Lactarius scrobiculatus (Scop. ex Fr.) Fr. | Picea | 2 | −27.3 | 0.6 |
| Lactarius theiogalus (Bull:Fr.) SF Gray | Promiscuous | 3 | −27.1 | 0.8 |
| Lactarius torminosus (Schff. ex Fr.) S.F. Gray | Betula | 3 | −25.8 | 1.4 |
| Lactarius trivialis Fr. | Promiscuous | 1 | −23.5 | |
| Lactarius uvidus Fr. | Betula (Salix) | 2 | −26.0 | 0.2 |
| Lactarius vietus Fr. | Betula (Salix) | 5 | −26.2 | 0.7 |
| Russula atrorubens Quél. | Conifers | 5 | −25.5 | 0.7 |
| Russula betularum Hora | Betula | 1 | −25.1 | |
| Russula coerulea Fr. | Pinus | 2 | −24.1 | 0.3 |
| Russula decolorans Fr. | Conifers | 5 | −25.4 | 0.3 |
| Russula emetica Fr. | Pinus (Picea) | 2 | −24.9 | 0.0 |
| Russula foetens Fr. | Promiscuous | 1 | −24.8 | |
| Russula gracillima Schaeff. | Betula | 1 | −27.7 | |
| Russula griseascens (Bon & Gaugué) L. Marti | Conifers | 1 | −26.4 | |
| Russula integra L. ex Fr. | (Picea) | 1 | −25.4 | |
| Russula paludosa Britz. | Conifers | 2 | −25.2 | 0.4 |
| Russula puellaris Fr. | Promiscuous | 1 | −25.5 | |
| Russula queletii Fr. | Picea | 1 | −27.5 | |
| Russula rhodopoda Zv. | Picea | 1 | −26.0 | |
| Russula sanguinea (Bull. ex St. am.) Fr. | Pinus (Picea) | 3 | −24.5 | 0.4 |
| Russula sardonia Fr. ex Rom. | Pinus | 2 | −26.3 | 1.7 |
| Russula vinosa Lindbl. | Conifers | 2 | −25.3 | 0.4 |
| Russula xerampelina (Schff. ex Secr.) Fr. | Pinus (Picea) | 1 | −26.3 | |
| Aphyllophorales | ||||
| Polyporaceae | ||||
| Albatrellus ovinus (Fr.) Kotl. & Pouz. | Conifers | 2 | −25.9 | 0.8 |
| Thelephoraceae | ||||
| Hydnellum ferrugineum (Fr.:Fr.) Karst. | Conifers | 2 | −22.5 | 0.5 |
| Hydnellum peckii Banker apud Peck | Conifers | 1 | −23.3 | |
| Phelloden niger (Fr.:Fr.) Karst. | Promiscuous | 1 | −22.7 | |
| Hydnaceae | ||||
| Hydnum repandum L.:Fr. | Promiscuous | 3 | −25.4 | 0.5 |
| Hydnum rufescens Fr. | Promiscuous | 6 | −25.4 | 1.3 |
| Cantharellaceae | ||||
| Cantharellus cibarius Fr. | Promiscuous | 3 | −26.3 | 0.3 |
| Cantharellus lutescens Fr. | Picea & Betula | 2 | −25.0 | 0.0 |
| Cantharellus tubaeformis Fr. | (Picea) | 4 | −25.2 | 0.6 |
| Saprophytic species | ||||
| Boletales | ||||
| Boletaceae | ||||
| Chalciporus piperatus (Bull. ex Fr.) Bat. | na | 2 | −22.0 | 0.9 |
| Agaricales | ||||
| Paxillaceae | ||||
| Hygrophoropsis aurantiaca (Wulf.:Fr.) Mre. | na | 2 | −21.6 | 1.1 |
| Paxillus atromentarius (Batsch: Fr.) Fr. | na | 1 | −23.0 | |
| Agaricales | ||||
| Tricholomataceae | ||||
| Clitocybe clavipes (Pers. ex Fr.) Kummer | na | 3 | −25.6 | 1.1 |
| Mycena pura (Pers. ex Fr.) Kummer | na | 1 | −22.9 | |
| Mycena rosella (Fr.) Kummer | na | 1 | −23.3 | |
| Entolomataceae | ||||
| Clitopilus prunulus (Scop. ex Fr.) Kummer | na | 2 | −22.9 | 0.1 |
| Entoloma nitidum Quél. | na | 3 | −23.5 | 0.4 |
| Rhodocybe nitellina (Fr.) Sing. | na | 1 | −20.8 | |
| Agaricaceae | ||||
| Agaricus haemorrhoideus Kalchbr. & Schulz. | na | 2 | −22.0 | 0.4 |
| Cystoderma carcharias (Pers. ex Secr.) Fay. | na | 1 | −23.8 | |
| Cortinariaceae | ||||
| Gymnopilus spectabilis (Fr.) Sing. | na | 2 | −24.0 | 3.1 |
| Aphyllophorales | ||||
| Auriscalpiaceae | ||||
| Auriscalpium vulgare S.F. Gray | na | 2 | −22.2 | 0.8 |
| Lycoperdaceae | ||||
| Lycoperdon foetidum Bonord. | na | 1 | −24.5 | |
The fungal fruit bodies and foliar samples from plants were dried (70°C, 24 h) and then were ground in a ball mill. Samples were analyzed for 13C abundance by using an online continuous flow CN analyzer coupled to an isotope mass spectrometer (18, 19). Results are expressed in the standard notation (δ13C) in parts per thousand (‰) relative to the international standard Vienna Pee Dee Belemnite, where δ13C = [(Rsample/Rstandard) − 1] × 1,000, and R is the molar ratio 13C/12C. The standard deviation based on analysis of replicated samples was 0.15‰. Linear two-source isotopic mixing models were used to calculate the fractional contribution of two C sources (plant hosts) to the C in conifer-specific and promiscuous fungi. For this, mean values of δ13C for host-specific fungi were used as isotopic endpoints.
RESULTS AND DISCUSSION.
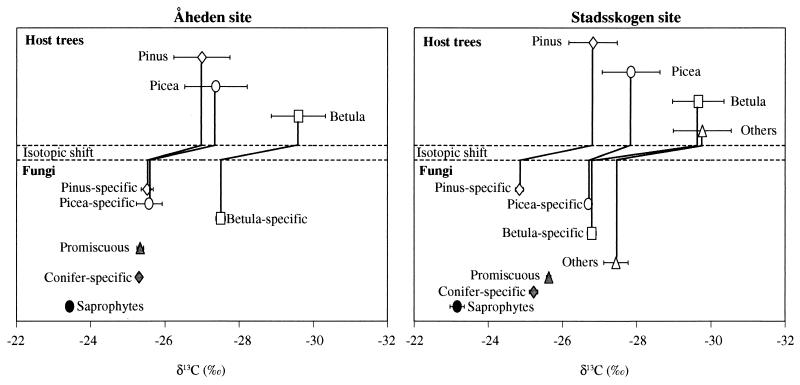
A total of 135 fungal species were sampled; 117 of these are ectomycorrhizal (Table 1). All groups of ectomycorrhizal fungi, i.e., host-specific and non-host-specific fungi, had a δ13C significantly different from that of saprophytic fungi (Tukey’s test, P < 0.05; Fig. 1). The difference between saprophytic and mycorrhizal fungi was found at both sites and is large enough to be developed as a tool to separate the two groups of fungi. For example, Chalciporus piperatus has been suspected to be mycorrhizal, but this has not been confirmed in experiments in which mycorrhizal syntheses have been attempted (20, 21). Its high 13C abundance at both sites (Table 1) would place this fungal species amongst the saprophytes. The difference in δ13C between tree foliage C and C in saprophytic fungi was ≈4‰ (Fig. 1), which was the difference between wood and fungal decomposers observed by Gleixner et al. (11).
Figure 1.
Natural abundance of 13C (δ13C) of fungal fruit bodies and of the host trees of ectomycorrhizal fungi (as indicated by connecting lines) in mixed temperate forests at two sites, Åheden and Stadsskogen, in Sweden. Promiscuous fungi are non-host-specific ectomycorrhizal fungi. Pine (P. sylvestris) is the dominant tree at both sites, most closely followed by spruce (P. abies) and birch (B. pendula). Other trees are A. incana, P. tremula, and S. caprea. Ectomycorrhizal fungi are arranged in groups according to their host specificity (compare with Table 1). Bars show SD of single observations. Replicates are individuals in case of host trees; in case of fungi, replicates are species (Table 1).
However, despite the significant differences between the mean δ13C values of saprophytic and mycorrhizal fungi, several ectomycorrhizal fungi had high δ13C (Table 1). These latter include Hydnellum ferrugineum, Hydnellum peckii, and Phellodon niger, all of which are known to be ectomycorrhizal (22). The fruit bodies of these three species are unique among the ectomycorrhizal fungi we sampled because they exist and grow for a much longer period than any of the other ectomycorrhizal fungi in our study. The mycorrhizal roots associated with fruit bodies of these three species are, as far as we know, also unique in that they appear to be degraded and moribund [carbonized sensu Agerer (22)]. The partly decomposed state of the mycorrhizal roots and the high 13C values of the fungal fruit bodies may suggest that the mutualistic balance between the host roots and the fungi may be weighed in favor of the fungi.
In both forests, the overstorey Scots pine (P. sylvestris L.) had, as predicted, the highest δ13C found among the tree species (Fig. 1) but was more or less closely followed by the other conifer, Norway spruce (Picea abies [L.] Karst.), which is the likely climax species at both sites. Understorey broadleaved species had up to 2–3‰ lower δ13C than the pines. Some of the ectomycorrhizal fungi collected are specific to one host species (Table 1), as shown by connecting lines in Fig. 1. Their δ13C was between 1.2 and 2.9‰ higher than that of their host species (Fig. 1). This isotopic shift may relate to fractionation during biochemical processes involved in the transfer of C from the host to the fungus, or it may suggest that such transfer occurs under conditions of higher rates of photosynthesis than the average (23). The isotopic shift between tree and fungus has to be taken into consideration but does not seriously confound the pattern of higher δ13C in fungi associated with overstorey trees.
Conifer-specific and promiscuous fungi, known to form ectomycorrhizal symbioses with a wide range of both coniferous and broadleaved species, had a δ13C, which indicated a large C flux from the overstorey pine. At the Åheden site (Fig. 1), there was no difference in δ13C between promiscuous fungi (eight species), conifer-specific fungi (eight species), and those specific for pine and spruce; that is, all (100%) of their C came from either of those two hosts or from both of them. At the Stadsskogen site (Fig. 1), simple isotopic mixing-model calculations indicated that pine contributed on average 57% of the C in promiscuous fungi (33 species) and 81% of the C in conifer-specific fungi (23 species). These calculations were based on the assumption that C comes from pine and spruce only in both cases; a contribution of C from understorey species other than spruce would increase the percent C from pine in promiscuous fungi! At Stadsskogen, three species of the commercially valuable genus Cantharellus (24) were sampled. Their δ13C values (Table 1) indicated that Cantharellus lutescens and Cantharellus tubaeformis received C from overstorey pine whereas Cantharellus cibarius, the famous golden chantarelle, also received C from understorey trees.
In conclusion, analysis of δ13C of fungi reveals whether they are saprophytic or ectomycorrhizal and, in the latter case, whether they receive C from overstorey or from understorey trees. It is interesting from an ecological perspective that the many promiscuous fungi (Table 1), which can associate with and connect both overstorey and understorey tree species, had a δ13C indicating a large C flux from overstorey trees. Such a flow of C from sunlit overstorey trees to a common ectomycorrhizal mycelium could act as a subsidy against the cost of the symbioses (2, 25, 26) in connected shaded understorey plants and could help them to survive a long period of shading.
Acknowledgments
We thank M. Sandström for preparing samples, H. Wallmark for mass spectrometric analysis, and R. Finlay, M. Högberg, and A. Jumpponen for comments. This research was supported by grants from the Swedish Natural Sciences Research Council and the Swedish Council for Forestry and Agricultural Research.
References
- 1.Hudson H J. Fungal Biology. London: Edward Arnold; 1986. [Google Scholar]
- 2.Smith S E, Read D J. Mycorrhizal Symbiosis. San Diego: Academic; 1997. [Google Scholar]
- 3.Gulden G, Hoiland K, Bendiksen K, Brandrud T E. Bibl Mycol. 1992;144:1–81. [Google Scholar]
- 4.Molina R, Massicotte H, Trappe J M. In: Mycorrhizal Functioning. Allen M F, editor. London: Chapman & Hall; 1992. pp. 357–423. [Google Scholar]
- 5.Väre H, Ohenoja E, Ohtonen R. Karstenia. 1996;36:1–18. [Google Scholar]
- 6.Trojanowski J, Haider K, Hüttermann A. Arch Microbiol. 1984;139:202–206. [Google Scholar]
- 7.Read D J, Francis R, Finlay R D. In: Ecological Interactions in Soil. Fitter A H, Atkinson D, Read D J, Usher M B, editors. Oxford: Blackwell; 1985. pp. 193–217. [Google Scholar]
- 8.Simard S W, Perry D A, Jones M D, Myrold D D, Durall D M, Molina R. Nature (London) 1997;388:579–582. [Google Scholar]
- 9.Perry D A, Margolis H, Choquette C, Molina R, Trappe J M. New Phytol. 1989;112:501–511. doi: 10.1111/j.1469-8137.1989.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnebrant K, Ek H, Finlay R D, Söderström B. New Phytol. 1993;124:231–242. doi: 10.1111/j.1469-8137.1993.tb03812.x. [DOI] [PubMed] [Google Scholar]
- 11.Gleixner G, Danier H-J, Werner R A, Schmidt H-L. Plant Physiol. 1993;102:1287–1290. doi: 10.1104/pp.102.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt H-L, Kexel H, Butzenlechner M, Schwarz S, Gleixner G, Thimet S, Werner R A, Gensler M. Stable Isotopes in the Biosphere. Kyoto: Kyoto Univ. Press; 1995. pp. 17–35. [Google Scholar]
- 13.Brooks J R, Flanagan L B, Buchmann N, Ehleringer J R. Oecologia. 1997;110:301–311. doi: 10.1007/s004420050163. [DOI] [PubMed] [Google Scholar]
- 14.Ehleringer J R, Field C B, Lin Z, Kuo C. Oecologia. 1986;70:520–526. doi: 10.1007/BF00379898. [DOI] [PubMed] [Google Scholar]
- 15.Farquhar G D, Ehleringer J R, Hubick H T. Annu Rev Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- 16.Hansen L, Knutsen H. Nordic Macromycetes, Vol. 2: Polyporales, Boletales, Agaricales, Russulales. Copenhagen: Nordsvamp; 1992. [Google Scholar]
- 17.Hansen L, Knutsen H. Nordic Macromycetes, Vol. 3: Heterobasidioid, Aphyllophoroid and Gasteromycetoid Basidiomycetes. Copenhagen: Nordsvamp; 1997. [Google Scholar]
- 18.Barrie A, Lemley M. Int Lab Techniques. 1989;19:82–91. [Google Scholar]
- 19.Ohlsson K E A, Wallmark P H. Analyst. 1999;124:571–577. [Google Scholar]
- 20.Alexander I J, Watling R. Proc R Soc Edinburgh. 1987;93B:107–115. [Google Scholar]
- 21.Godbout C, Fortin J A. New Phytol. 1983;94:349–262. [Google Scholar]
- 22.Agerer R. Color Atlas of Ectomycorrhizae. Schwäbisch Gmünd, Germany: Einhorn-Verlag; 1987–1993. [Google Scholar]
- 23.Pate J, Arthur D. Oecologia. 1998;117:301–311. doi: 10.1007/s004420050663. [DOI] [PubMed] [Google Scholar]
- 24.Watling R. Nature (London) 1997;385:299–300. [Google Scholar]
- 25.Finlay R D, Söderström B. In: Mycorrhizal Functioning. Allen M F, editor. London: Chapman & Hall; 1992. pp. 134–160. [Google Scholar]
- 26.Rygiewicz P T, Anderson C P. Nature (London) 1996;369:58–60. [Google Scholar]