Abstract
Background: In rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), patients demonstrate low levels of adrenal hormones.
Objective: To investigate whether increased renal clearance and daily excretion contribute to this phenomenon.
Methods: Thirty patients with RA, 32 with SLE, and 54 healthy subjects (HS) participated. Serum and urinary levels of cortisol, cortisone, 17-hydroxyprogesterone (17OHP), androstenedione, dehydroepiandrosterone (DHEA), and DHEA sulphate (DHEAS) were measured.
Results: Clearance of DHEAS and DHEA was lower in patients than in HS, and clearance of androstenedione was somewhat higher in patients than in HS, but daily excretion of this latter hormone was low. Clearance of cortisol, cortisone, and 17OHP was similar between the groups. The total molar amount per hour of excreted DHEA, DHEAS, and androstenedione was lower in patients than HS (but similar for cortisol). Serum DHEAS levels correlated with urinary DHEAS levels in HS and patients, whereby HS excreted 5–10 times more of this hormone than excreted by patients. Low serum levels of adrenal androgens and cortisol in patients as compared with HS were confirmed, and proteinuria was not associated with changes of measured renal parameters.
Conclusions: This study in patients with RA and SLE demonstrates that low serum levels of adrenal androgens and cortisol are not due to increased renal clearance and daily loss of these hormones. Decreased adrenal production or increased conversion or conjugation to downstream hormones are the most likely causes of inadequately low serum levels of adrenal hormones in RA and SLE.
Full Text
The Full Text of this article is available as a PDF (242.5 KB).
Figure 1.
Cascades of adrenal steroidogenesis. A line with an arrow at the end indicates that the respective mediator stimulates the enzyme step (ACTH, IL6), a line with a bar at the end demonstrates that the respective mediator inhibits the enzyme step (IL1, TNF, TGFß). Enzymes and abbreviations: 1, P450scc = side chain cleavage enzyme; 2, 3ß-hydroxysteroid dehydrogenase; 3, P450c21 = 21α-hydroxylase; 4, P450c11 = 11ß-hydroxylase; 5 and 6, P450c17 = 17α-hydroxylase and 17/20-lyase (double enzyme step); 7, DHEA sulphotransferase and DHEAS sulphatase; 8, 11ß-hydroxysteroid dehydrogenase types I and II; 9, aromatase complex; ACTH, adrenocorticotrophic hormone; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulphate; IL1, interleukin 1; IL6, interleukin 6; TGFß, transforming growth factor ß1; TNF, tumour necrosis factor.
Figure 2.

Serum hormone concentration of adrenal steroid hormones. (A-C) Data of patients with RA; (D-F) results of patients with SLE. Data are given as means (SEM). *p<0.05; †p<0.005; ‡p<0.001 for the comparison of the respective group with HS. CSN, cortisone; DHEAS, DHEA sulphate; 17OHP, 17-hydroxyprogesterone; ASD, androstenedione; DHEA, dehydroepiandrosterone.
Figure 3.
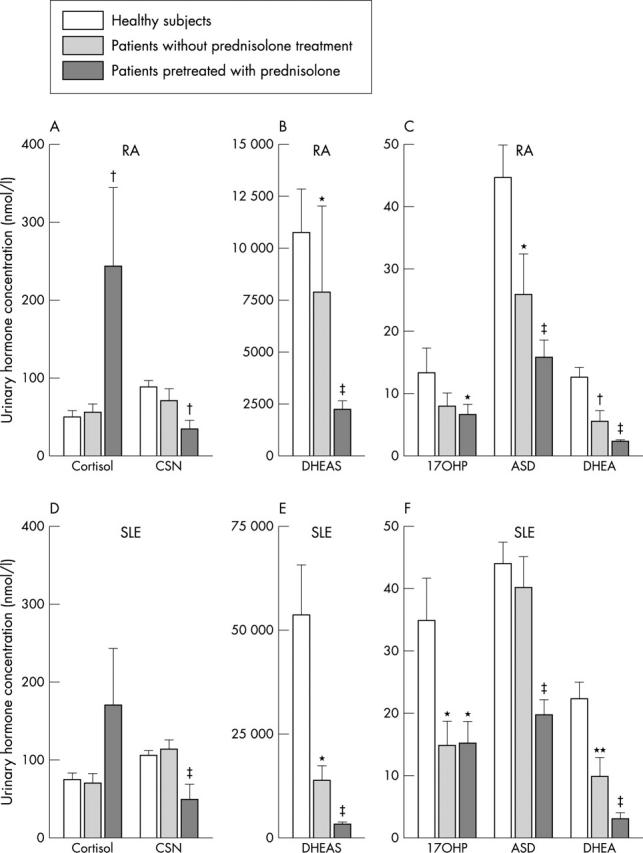
Urinary hormone concentration of adrenal steroid hormones. (A-C) Data of patients with RA; (D-F) results of patients with SLE. Data are given as means (SEM). *p<0.05; **p<0.01; †p<0.005; ‡p<0.001 for the comparison of the respective group with HS. For abbreviations see the legend to fig 2.
Figure 4.
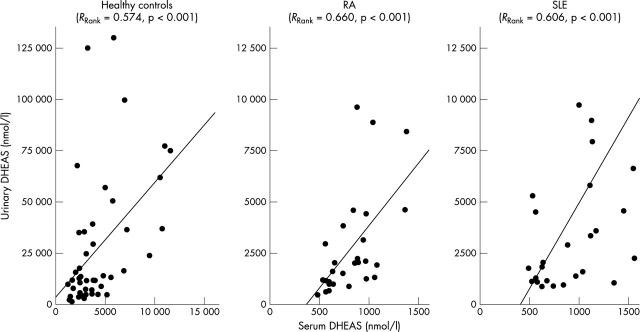
Interrelation between serum DHEAS and urinary DHEAS in HS, patients with RA, and patients with SLE, including those with prednisolone treatment. The x axis and the y axis were adjusted to the different numerical levels because the HS had clearly higher serum and urine concentrations than both patient groups. The panels include the linear regression line, the Spearman rank correlation coefficient, and the respective p value.
Figure 5.

Substance clearance of adrenal steroid hormones. (A) Data of patients with RA; (B) results of patients with SLE. Open bars denote healthy subjects, light grey bars represent patients without prednisolone treatment, and dark grey bars demonstrate data of prednisolone pretreated patients. Data are given as means (SEM). *p<0.05; **p<0.01; †p<0.005; ‡p<0.001 for the comparison of the respective group versus HS. Crea, creatinine; for other abbreviations see the legend to fig 2,
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Klebl F., Rogler G., Bregenzer N., Schölmerich J., Straub R. H. Patients with refractory Crohn's disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409–414. doi: 10.1046/j.1365-2036.2003.01433.x. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Castagnetta Luigi A., Carruba Giuseppe, Granata Orazia M., Stefano Rosalba, Miele Monica, Schmidt Martin, Cutolo Maurizio, Straub Rainer H. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003 Dec;30(12):2597–2605. [PubMed] [Google Scholar]
- Chang Deh-Ming, Lan Joung-Liang, Lin Hsiao-Yi, Luo Shue-Fen. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002 Nov;46(11):2924–2927. doi: 10.1002/art.10615. [DOI] [PubMed] [Google Scholar]
- Crofford L. J., Kalogeras K. T., Mastorakos G., Magiakou M. A., Wells J., Kanik K. S., Gold P. W., Chrousos G. P., Wilder R. L. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997 Apr;82(4):1279–1283. doi: 10.1210/jcem.82.4.3852. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Balleari E., Giusti M., Monachesi M., Accardo S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonadotropin stimulation. Arthritis Rheum. 1988 Oct;31(10):1314–1317. doi: 10.1002/art.1780311015. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Foppiani L., Prete C., Ballarino P., Sulli A., Villaggio B., Seriolo B., Giusti M., Accardo S. Hypothalamic-pituitary-adrenocortical axis function in premenopausal women with rheumatoid arthritis not treated with glucocorticoids. J Rheumatol. 1999 Feb;26(2):282–288. [PubMed] [Google Scholar]
- Deighton C. M., Watson M. J., Walker D. J. Sex hormones in postmenopausal HLA-identical rheumatoid arthritis discordant sibling pairs. J Rheumatol. 1992 Nov;19(11):1663–1667. [PubMed] [Google Scholar]
- Demir H., Keleştimur F., Tunç M., Kirnap M., Ozügül Y. Hypothalamo-pituitary-adrenal axis and growth hormone axis in patients with rheumatoid arthritis. Scand J Rheumatol. 1999;28(1):41–46. doi: 10.1080/03009749950155779. [DOI] [PubMed] [Google Scholar]
- Fehér G. K., Fehér T., Zahumenszky Z. Study on the inactivation mechanism of androgens in rheumatoid arthritis: excretory rate of free and conjugated 17-ketosteroids. Endokrinologie. 1979 Apr;73(2):167–172. [PubMed] [Google Scholar]
- Gudbjörnsson B., Skogseid B., Oberg K., Wide L., Hällgren R. Intact adrenocorticotropic hormone secretion but impaired cortisol response in patients with active rheumatoid arthritis. Effect of glucocorticoids. J Rheumatol. 1996 Apr;23(4):596–602. [PubMed] [Google Scholar]
- Gutiérrez M. A., García M. E., Rodriguez J. A., Mardonez G., Jacobelli S., Rivero S. Hypothalamic-pituitary-adrenal axis function in patients with active rheumatoid arthritis: a controlled study using insulin hypoglycemia stress test and prolactin stimulation. J Rheumatol. 1999 Feb;26(2):277–281. [PubMed] [Google Scholar]
- Hall J., Morand E. F., Medbak S., Zaman M., Perry L., Goulding N. J., Maddison P. J., O'Hare J. P. Abnormal hypothalamic-pituitary-adrenal axis function in rheumatoid arthritis. Effects of nonsteroidal antiinflammatory drugs and water immersion. Arthritis Rheum. 1994 Aug;37(8):1132–1137. doi: 10.1002/art.1780370804. [DOI] [PubMed] [Google Scholar]
- Hedman M., Nilsson E., de la Torre B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin Exp Rheumatol. 1992 Jan-Feb;10(1):25–30. [PubMed] [Google Scholar]
- Johnson E. O., Vlachoyiannopoulos P. G., Skopouli F. N., Tzioufas A. G., Moutsopoulos H. M. Hypofunction of the stress axis in Sjögren's syndrome. J Rheumatol. 1998 Aug;25(8):1508–1514. [PubMed] [Google Scholar]
- Judd A. M., Call G. B., Barney M., McIlmoil C. J., Balls A. G., Adams A., Oliveira G. K. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann N Y Acad Sci. 2000;917:628–637. doi: 10.1111/j.1749-6632.2000.tb05428.x. [DOI] [PubMed] [Google Scholar]
- Jättelä M., Ilvesmäki V., Voutilainen R., Stenman U. H., Saksela E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin-induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology. 1991 Jan;128(1):623–629. doi: 10.1210/endo-128-1-623. [DOI] [PubMed] [Google Scholar]
- Kanik K. S., Chrousos G. P., Schumacher H. R., Crane M. L., Yarboro C. H., Wilder R. L. Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab. 2000 Apr;85(4):1461–1466. doi: 10.1210/jcem.85.4.6534. [DOI] [PubMed] [Google Scholar]
- Ligthart G. J., Corberand J. X., Fournier C., Galanaud P., Hijmans W., Kennes B., Müller-Hermelink H. K., Steinmann G. G. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984 Nov;28(1):47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- Masi A. T., Josipović D. B., Jefferson W. E. Low adrenal androgenic-anabolic steroids in women with rheumatoid arthritis (RA): gas-liquid chromatographic studies of RA patients and matched normal control women indicating decreased 11-deoxy-17-ketosteroid excretion. Semin Arthritis Rheum. 1984 Aug;14(1):1–23. doi: 10.1016/0049-0172(84)90005-2. [DOI] [PubMed] [Google Scholar]
- Mateo L., Nolla J. M., Bonnin M. R., Navarro M. A., Roig-Escofet D. Sex hormone status and bone mineral density in men with rheumatoid arthritis. J Rheumatol. 1995 Aug;22(8):1455–1460. [PubMed] [Google Scholar]
- Mirone L., Altomonte L., D'Agostino P., Zoli A., Barini A., Magaro M. A study of serum androgen and cortisol levels in female patients with rheumatoid arthritis. Correlation with disease activity. Clin Rheumatol. 1996 Jan;15(1):15–19. doi: 10.1007/BF02231678. [DOI] [PubMed] [Google Scholar]
- Petri Michelle A., Lahita Robert G., Van Vollenhoven Ronald F., Merrill Joan T., Schiff Michael, Ginzler Ellen M., Strand Vibeke, Kunz Arlene, Gorelick Kenneth J., Schwartz Kenneth E. Effects of prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2002 Jul;46(7):1820–1829. doi: 10.1002/art.10364. [DOI] [PubMed] [Google Scholar]
- Reincke M., Heppner C., Petzke F., Allolio B., Arlt W., Mbulamberi D., Siekmann L., Vollmer D., Winkelmann W., Chrousos G. P. Impairment of adrenocortical function associated with increased plasma tumor necrosis factor-alpha and interleukin-6 concentrations in African trypanosomiasis. Neuroimmunomodulation. 1994 Jan;1(1):14–22. doi: 10.1159/000095930. [DOI] [PubMed] [Google Scholar]
- Rovensky J., Imrich R., Koska J., Kovalancik M., Killinger Z., Payer J., Vigas M., Jezova D. Cortisol elimination from plasma in premenopausal women with rheumatoid arthritis. Ann Rheum Dis. 2003 Jul;62(7):674–676. doi: 10.1136/ard.62.7.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook P. N., Eisman J. A., Champion G. D., Pocock N. A. Sex hormone status and osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum. 1988 Aug;31(8):973–978. doi: 10.1002/art.1780310805. [DOI] [PubMed] [Google Scholar]
- Santos-Montes A., Gonzalo-Lumbreras R., Izquierdo-Hornillos R. Simultaneous determination of cortisol and cortisone in urine by reversed-phase high-performance liquid chromatography. Clinical and doping control applications. J Chromatogr B Biomed Appl. 1995 Nov 3;673(1):27–33. doi: 10.1016/0378-4347(95)00253-f. [DOI] [PubMed] [Google Scholar]
- Solerte S. B., Severgnini S., Locatelli M., Cerutti N., Rondanelli M., Netti M. A., Ferrari E., Fioravanti M. Nephelometry in the clinical assessment of glomerular proteinuria and tubular function in diabetic nephropathy. Clin Nephrol. 1997 Sep;48(3):151–158. [PubMed] [Google Scholar]
- Straub R. H., Cutolo M. Involvement of the hypothalamic--pituitary--adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic pathogenetic role. Arthritis Rheum. 2001 Mar;44(3):493–507. doi: 10.1002/1529-0131(200103)44:3<493::AID-ANR95>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Straub R. H., Konecna L., Hrach S., Rothe G., Kreutz M., Schölmerich J., Falk W., Lang B. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab. 1998 Jun;83(6):2012–2017. doi: 10.1210/jcem.83.6.4876. [DOI] [PubMed] [Google Scholar]
- Straub Rainer H., Paimela Leena, Peltomaa Ritva, Schölmerich Jürgen, Leirisalo-Repo Marjatta. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 2002 Mar;46(3):654–662. doi: 10.1002/art.10177. [DOI] [PubMed] [Google Scholar]
- Straub Rainer H., Pongratz Georg, Schölmerich Jürgen, Kees Frieder, Schaible Thomas F., Antoni Christian, Kalden Joachim R., Lorenz Hanns-Martin. Long-term anti-tumor necrosis factor antibody therapy in rheumatoid arthritis patients sensitizes the pituitary gland and favors adrenal androgen secretion. Arthritis Rheum. 2003 Jun;48(6):1504–1512. doi: 10.1002/art.11036. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Templ E., Koeller M., Riedl M., Wagner O., Graninger W., Luger A. Anterior pituitary function in patients with newly diagnosed rheumatoid arthritis. Br J Rheumatol. 1996 Apr;35(4):350–356. doi: 10.1093/rheumatology/35.4.350. [DOI] [PubMed] [Google Scholar]
- Valentino R., Savastano S., Tommaselli A. P., Riccio A., Mariniello P., Pronesti G., De Divitiis P. M., Lombardi G. Hormonal pattern in women affected by rheumatoid arthritis. J Endocrinol Invest. 1993 Sep;16(8):619–624. doi: 10.1007/BF03347683. [DOI] [PubMed] [Google Scholar]
- Zietz B., Reber T., Oertel M., Glück T., Schölmerich J., Straub R. H. Altered function of the hypothalamic stress axes in patients with moderately active systemic lupus erythematosus. II. Dissociation between androstenedione, cortisol, or dehydroepiandrosterone and interleukin 6 or tumor necrosis factor. J Rheumatol. 2000 Apr;27(4):911–918. [PubMed] [Google Scholar]
- van Vollenhoven R. F., Engleman E. G., McGuire J. L. Dehydroepiandrosterone in systemic lupus erythematosus. Results of a double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheum. 1995 Dec;38(12):1826–1831. doi: 10.1002/art.1780381216. [DOI] [PubMed] [Google Scholar]
- van den Brink H. R., Blankenstein M. A., Koppeschaar H. P., Bijlsma J. W. Influence of disease activity on steroid hormone levels in peripheral blood of patients with rheumatoid arthritis. Clin Exp Rheumatol. 1993 Nov-Dec;11(6):649–652. [PubMed] [Google Scholar]