Abstract
Objective: To evaluate the presence of histones and nucleosomes in cell lysates of freshly isolated peripheral blood mononuclear cells (PBMC), fully activated lymphoblasts, or lymphoblasts after induction of apoptosis.
Methods: Each histone class (H1, H2A, H2B, H3, and H4) was detected by western blot analysis with specific antibodies. Annexin V/propidium iodide (PI) staining was performed for each treatment to distinguish viable, early, and late apoptotic, and necrotic cells. DNA degradation was evaluated by PI staining in a hypotonic buffer.
Results: All five histones were detected in cell lysates of activated lymphoblasts in higher concentrations than in lysates of PBMC. An additional significant increase of H2A, H2B, H3, H4, and complete nucleosomes in cell lysates of lymphoblasts was found during interleukin (IL)2 deprivation for 8 or 24 hours. The content of these histones or nucleosomes in cell lysates decreased after delayed IL2 readdition. H1 content in cell lysates was not affected by IL2 deprivation or addition. Quantities of H2A, H2B, H3, and H4 in cell lysates correlated significantly with signs of early apoptosis. UV-B light exposure or etoposide treatment of lymphoblasts resulted in a distinct increase for each histone class in cell lysates compared with standard curves. No bands for post-translationally modified histones were detected in cell lysates in contrast with signals in nuclear preparations.
Conclusion: Extranuclear accumulation of histones and nucleosomes is an early event of apoptosis in human lymphoblasts. Dysregulation of early apoptosis might support the induction of autoimmunity against nuclear components.
Full Text
The Full Text of this article is available as a PDF (360.7 KB).
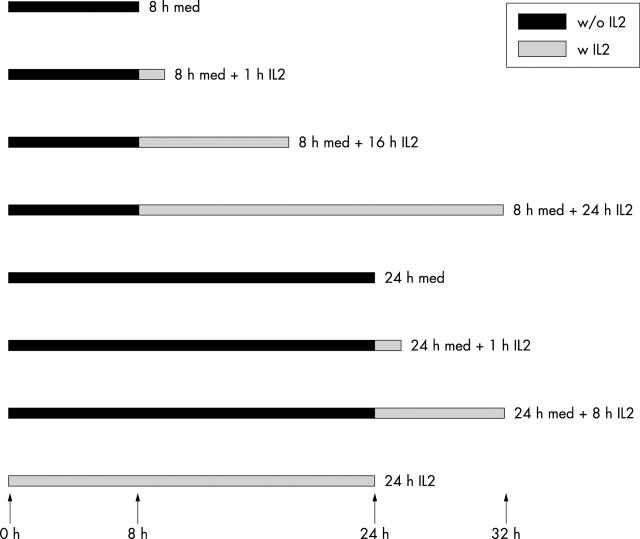
Figure 1.
Experimental design showing times of IL2 withdrawal and readdition. Black bars represent the time of IL2 deprivation (med = medium R10); grey bars represent the time of the treatment with 10 U/ml IL2.
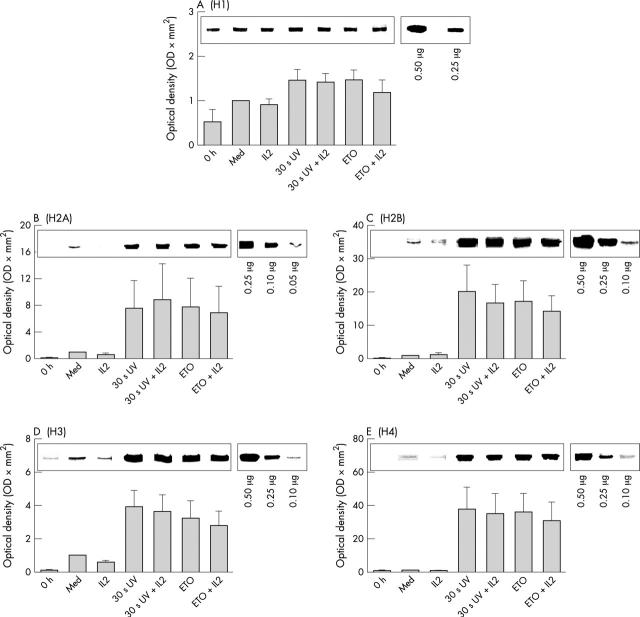
Figure 5.
Densitometric analysis of histone H1 (A), H2A (B), H2B (C), H3 (D), and H4 (E) in cell lysates of lymphoblasts after induced apoptosis. Cells were lysed, and 40 µg of cell lysates was separated using SDS-PAGE as described in "Material and methods". Western blot was performed with monoclonal antibodies directed against distinct histones. Band intensities were scanned and analysed by densitometry. The corresponding integrated absorbance (mean (SEM); n = 6 normal healthy donors) is depicted in relation to the band intensity of the histone band at 8 hours of cytokine withdrawal (8 hours med). A representative example of histone bands is illustrated. ETO, etoposide.
Figure 2.
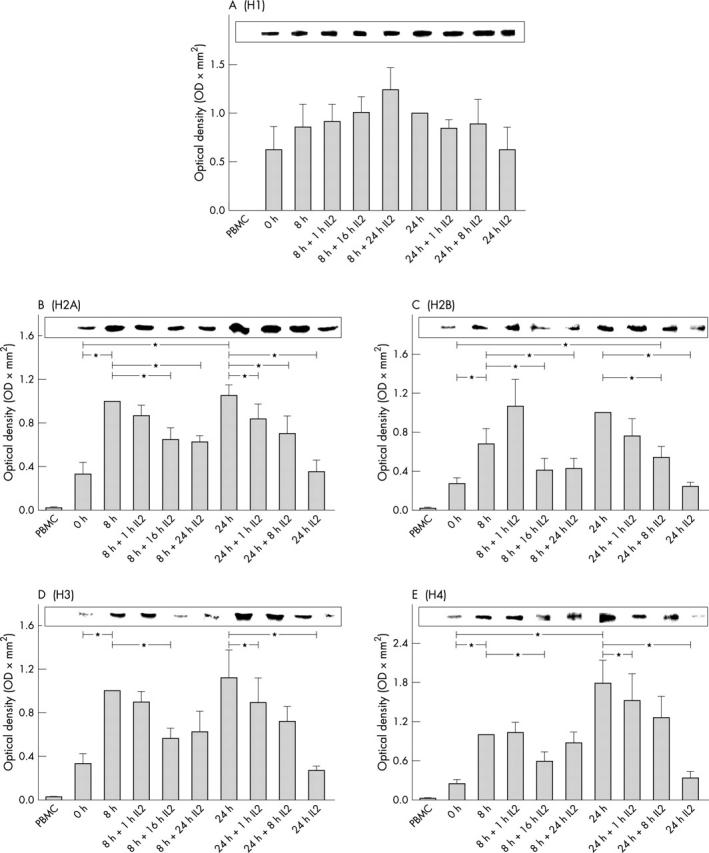
Densitometric analysis of histone H1 (A), H2A (B), H2B (C), H3 (D), and H4 (E) in cell lysates of PBMC (first column) and lymphoblasts (all other columns). Cells were lysed, 40 µg of cell lysates were separated using SDS-PAGE as described in "Material and methods". Western blot was performed with monoclonal antibodies directed against distinct histones. Band intensities were scanned and analysed by densitometry. The corresponding integrated absorbance (mean (SEM); n = 6 normal healthy donors) is depicted in relation to the band intensity of the histone band at time 8 (B, D, E) or 24 hours (A, C) of cytokine withdrawal. A representative example of histone bands is illustrated. *Indicates significant difference (p<0.05) between the different times. In addition, the amount of each histone class in cell lysates of PBMC was significantly lower (p<0.001) than in cell lysates of lymphoblasts at all times.
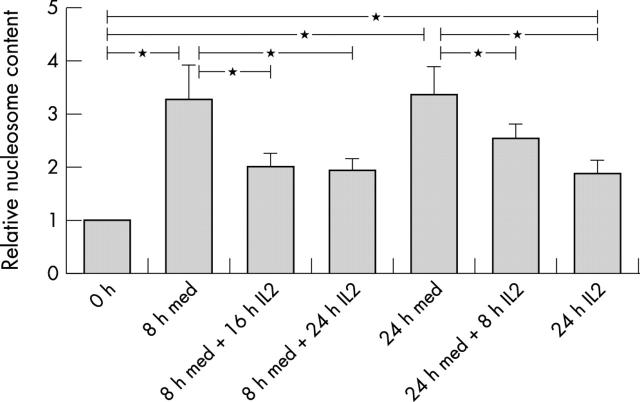
Figure 3.
Relative contents of nucleosomes in cell lysates of apoptotic lymphoblasts compared with lymphoblasts at t0. Nucleosome ELISA was performed as described in "Material and methods". Absorption values (405 nm) were referred to lymphoblasts at t0 (value of 1). Data are mean (SEM) of six normal healthy donors. *Indicates significant difference (p<0.05) between the different times.
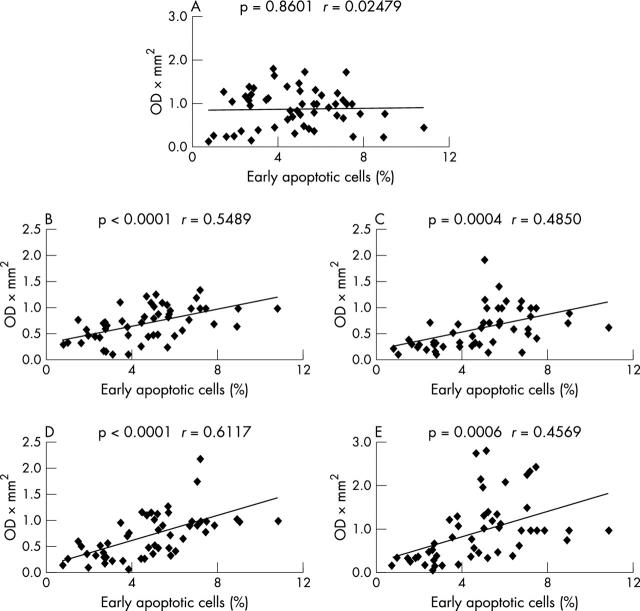
Figure 4.
Correlation of integrated optical density (ODxmm2) of histone bands H1 (A), H2A (B), H2B (C), H3 (D), and H4 (E) with the percentage of early apoptotic cells. The normalised values of the band intensities for each histone after each treatment were correlated with the percentage of early apoptotic cells (AxVposPIneg) after identical treatment within the identical experiment. The p values and the correlation coefficients r are given.
Figure 6.
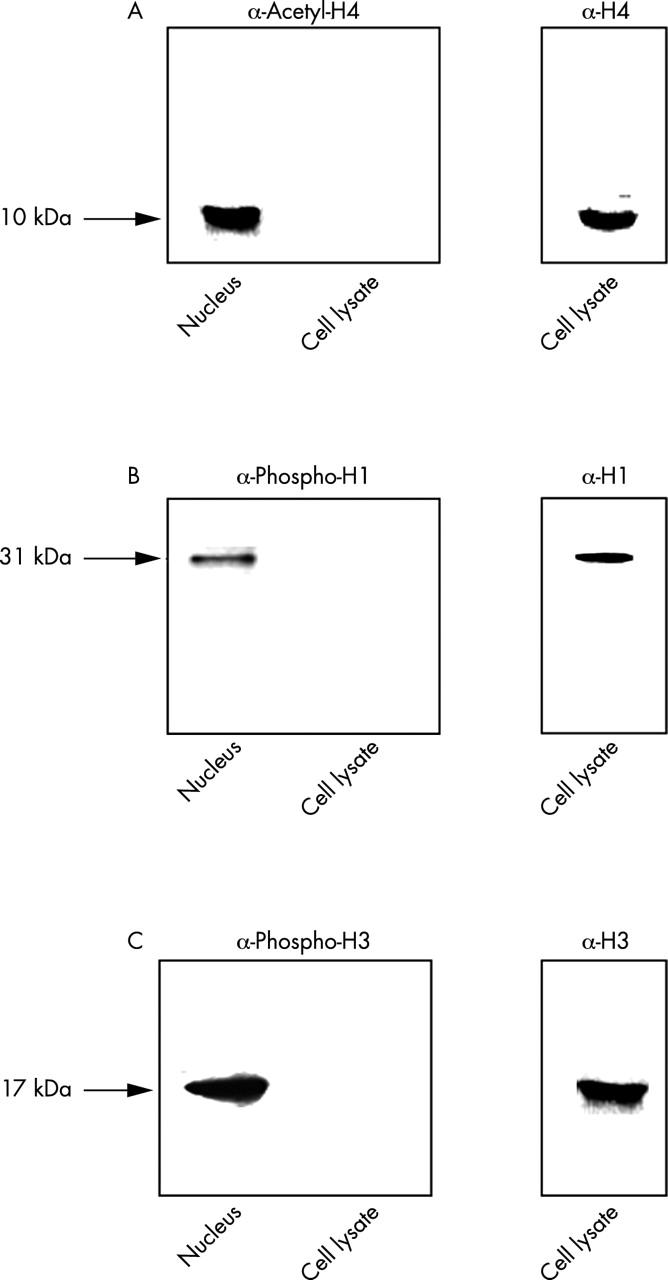
Western blots for the detection of the following post-translationally modified histones: (A) acetylated H4; (B) phosphorylated H1; and (c) phosphorylated H3. Cells were lysed and 40 µg of cell lysates or 1/10 of the nuclear preparation from 2x106 lymphoblasts was separated using SDS-PAGE as described in "Material and methods". Western blot was performed with antibodies directed against the distinct post-translational modifications of the specific histone. As controls, the identical cell lysates were separated in a different gel. Western blot was performed with monoclonal antibodies directed against H4 (A; KM2), H1 (B; MRA12), or H3 (C; LG 2.1.).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berden J. H., Licht R., van Bruggen M. C., Tax W. J. Role of nucleosomes for induction and glomerular binding of autoantibodies in lupus nephritis. Curr Opin Nephrol Hypertens. 1999 May;8(3):299–306. doi: 10.1097/00041552-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Blank N., Kriegel M., Hieronymus T., Geiler T., Winkler S., Kalden J. R., Lorenz H. M. CD45 tyrosine phosphatase controls common gamma-chain cytokine-mediated STAT and extracellular signal-related kinase phosphorylation in activated human lymphoblasts: inhibition of proliferation without induction of apoptosis. J Immunol. 2001 May 15;166(10):6034–6040. doi: 10.4049/jimmunol.166.10.6034. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L., Andrade F., Ulanet D., Wong W. B., Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999 Sep 20;190(6):815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995 Jun;7(3):337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Eckert A., Oster M., Zerfass R., Hennerici M., Müller W. E. Elevated levels of fragmented DNA nucleosomes in native and activated lymphocytes indicate an enhanced sensitivity to apoptosis in sporadic Alzheimer's disease. Specific differences to vascular dementia. Dement Geriatr Cogn Disord. 2001 Mar-Apr;12(2):98–105. doi: 10.1159/000051242. [DOI] [PubMed] [Google Scholar]
- Emlen W., Niebur J., Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994 Apr 1;152(7):3685–3692. [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998 Jan 1;391(6662):43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Gaipl U. S., Kuenkele S., Voll R. E., Beyer T. D., Kolowos W., Heyder P., Kalden J. R., Herrmann M. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001 Apr;8(4):327–334. doi: 10.1038/sj.cdd.4400826. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Thomas J. O. Antibodies to histones in systemic lupus erythematosus: localization of prominent autoantigens on histones H1 and H2B. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7410–7414. doi: 10.1073/pnas.80.24.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Voll R. E., Zoller O. M., Hagenhofer M., Ponner B. B., Kalden J. R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998 Jul;41(7):1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Huggins M. L., Todd I., Cavers M. A., Pavuluri S. R., Tighe P. J., Powell R. J. Antibodies from systemic lupus erythematosus (SLE) sera define differential release of autoantigens from cell lines undergoing apoptosis. Clin Exp Immunol. 1999 Nov;118(2):322–328. doi: 10.1046/j.1365-2249.1999.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda S., Kanayama Y., Okamura M., Amatsu K., Negoro N., Takeda T., Inoue T. Clinical significance of antibodies to histones in systemic lupus erythematosus. J Rheumatol. 1989 Jan;16(1):24–28. [PubMed] [Google Scholar]
- Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994 Sep 1;84(5):1415–1420. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lecoeur Hervé. Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp Cell Res. 2002 Jul 1;277(1):1–14. doi: 10.1006/excr.2002.5537. [DOI] [PubMed] [Google Scholar]
- Lorenz H. M., Grünke M., Hieronymus T., Herrmann M., Kühnel A., Manger B., Kalden J. R. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. 1997 Feb;40(2):306–317. doi: 10.1002/art.1780400216. [DOI] [PubMed] [Google Scholar]
- Lorenz H. M., Herrmann M., Winkler T., Gaipl U., Kalden J. R. Role of apoptosis in autoimmunity. Apoptosis. 2000 Nov;5(5):443–449. doi: 10.1023/a:1009692902805. [DOI] [PubMed] [Google Scholar]
- Lorenz Hanns-Martin, Grünke Mathias, Hieronymus Thomas, Winkler Silke, Blank Norbert, Rascu Astrid, Wendler Jörg, Geiler Thomas, Kalden Joachim R. Hyporesponsiveness to gammac-chain cytokines in activated lymphocytes from patients with systemic lupus erythematosus leads to accelerated apoptosis. Eur J Immunol. 2002 May;32(5):1253–1263. doi: 10.1002/1521-4141(200205)32:5<1253::AID-IMMU1253>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lu L., Kaliyaperumal A., Boumpas D. T., Datta S. K. Major peptide autoepitopes for nucleosome-specific T cells of human lupus. J Clin Invest. 1999 Aug;104(3):345–355. doi: 10.1172/JCI6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor F., Cohen I. R. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J Immunol. 1996 Jan 15;156(2):515–522. [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Perniok A., Wedekind F., Herrmann M., Specker C., Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7(2):113–118. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- Rosen A., Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999 Jan;6(1):6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- Salgame P., Varadhachary A. S., Primiano L. L., Fincke J. E., Muller S., Monestier M. An ELISA for detection of apoptosis. Nucleic Acids Res. 1997 Feb 1;25(3):680–681. doi: 10.1093/nar/25.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Voll R. E., Roth E. A., Girkontaite I., Fehr H., Herrmann M., Lorenz H. M., Kalden J. R. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997 Dec;40(12):2162–2171. doi: 10.1002/art.1780401210. [DOI] [PubMed] [Google Scholar]
- Wu Dongcheng, Ingram Alistair, Lahti Jill H., Mazza Brie, Grenet Jose, Kapoor Anil, Liu Lieqi, Kidd Vincent J., Tang Damu. Apoptotic release of histones from nucleosomes. J Biol Chem. 2002 Jan 25;277(14):12001–12008. doi: 10.1074/jbc.M109219200. [DOI] [PubMed] [Google Scholar]
- Zubiaga A. M., Munoz E., Huber B. T. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992 Jul 1;149(1):107–112. [PubMed] [Google Scholar]