Abstract
Objective: To assess longitudinal expression of a proliferation-inducing ligand (APRIL) in patients with systemic lupus erythematosus (SLE) and its correlation with B lymphocyte stimulator (BLyS) expression, serum anti-dsDNA titres, and clinical disease activity.
Methods: Sixty eight patients with SLE were longitudinally followed up for a median of 369 days. At each visit the physician assessed disease activity by SLEDAI, and blood was collected for determination of serum APRIL and BLyS levels and of blood APRIL and BLyS mRNA levels. Fifteen normal control subjects underwent similar laboratory evaluation.
Results: Dysregulation of APRIL was not as great as that of BLyS. Changes in serum levels of APRIL and BLyS over time were usually discordant, whereas blood levels of APRIL and BLyS mRNA strongly paralleled each other. Serum APRIL levels modestly, but significantly, inversely correlated with serum anti-dsDNA titres in anti-dsDNA positive patients analysed in aggregate. Moreover, serum APRIL levels modestly, but significantly, inversely correlated with clinical disease activity in all patients analysed in aggregate.
Conclusion: Serum levels of APRIL and BLyS are differentially regulated. APRIL may serve as a down modulator of serological and/or clinical autoimmunity in patients with SLE. This may have important ramifications for BLyS targeted treatment, and it remains to be determined whether agents which neutralise only BLyS will be preferable to agents which neutralise both BLyS and APRIL.
Full Text
The Full Text of this article is available as a PDF (153.6 KB).
Figure 1.
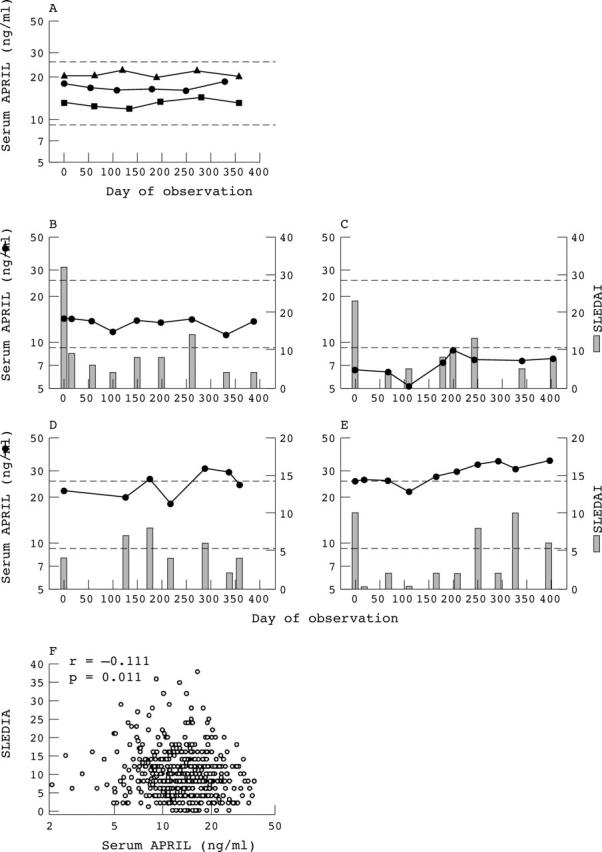
Heterogeneity in serum APRIL phenotypes among patients with SLE and relation to disease activity. (A) Serum samples collected from three representative normal controls (circles, triangles, squares) were assayed for APRIL levels. By definition, the first serum samples were collected on day 0. The dashed lines indicate 2SD above and below the geometric mean of all the values (n = 86) obtained from the 15 control subjects and arbitrarily define the "normal" range. (B-E) Serum samples collected from individual patients with SLE were assayed for APRIL levels (circles). Disease activity was measured by SLEDAI (bars). Results for representative patients exhibiting the "persistently normal" serum APRIL phenotype (B), the "persistently low" serum APRIL phenotype (C), the "intermittently raised" serum APRIL phenotype (D), or the "persistently raised" serum APRIL phenotype (E) are illustrated. (F) Serum APRIL levels plotted against the corresponding SLEDAI scores for the 523 individual serum samples from the 68 patients with SLE for whom serum APRIL levels and SLEDAI scores were both determined.
Figure 2.
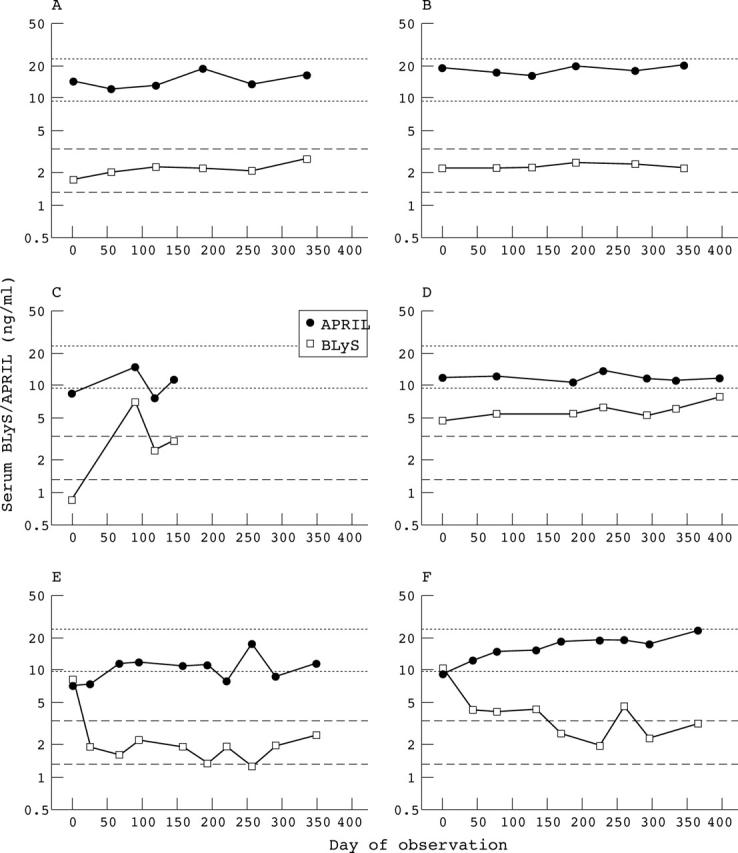
Relation between serum APRIL and serum BLyS levels. Serum samples collected from two representative normal controls (A, B) and four representative patients with SLE (C–F) were assayed for APRIL and BLyS levels. The dotted lines delineate the normal range for serum APRIL, and the dashed lines delineate the normal range for serum BLyS.15
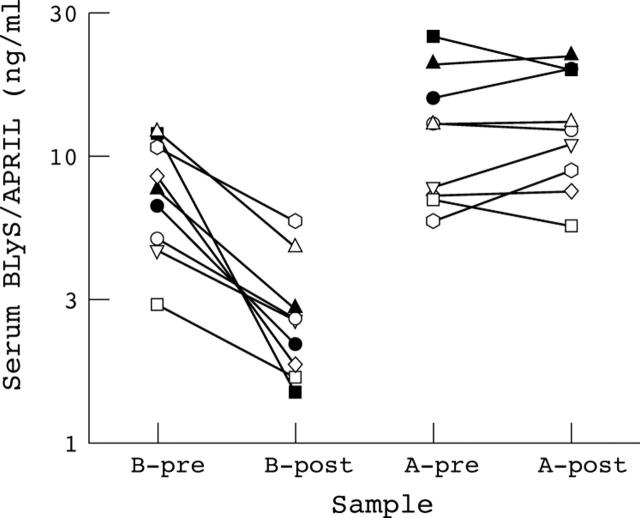
Figure 3.
Insensitivity of serum APRIL levels to treatment with high dose corticosteroids. Nine patients with SLE were treated with short courses of high dose corticosteroids.15 BLyS levels in pretreatment (B-pre) and post-treatment (B-post) serum samples and APRIL levels in pretreatment (A-pre) and post-treatment (A-post) sera are plotted.
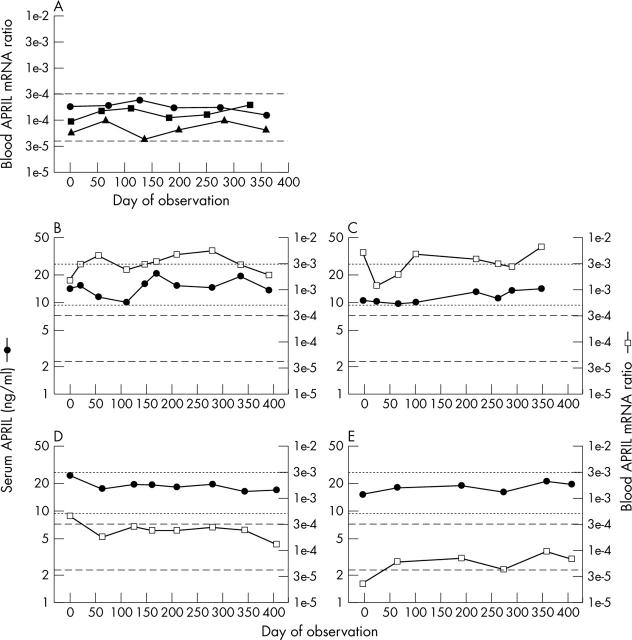
Figure 4.
Heterogeneity in blood APRIL mRNA phenotypes among patients with SLE. (A) Peripheral blood samples collected from the same three representative normal controls illustrated in fig 1A (circles, triangles, squares) were assayed for APRIL mRNA levels. By definition, the first blood samples were collected on day 0. The dashed lines indicate 2SD above and below the geometric mean of all the values (n = 88) obtained from the 15 control subjects and arbitrarily define the "normal" range. (B–E) Serum APRIL levels (filled circles) and blood APRIL mRNA levels (open squares) were measured. The dotted lines delineate the normal range for serum APRIL, and the dashed lines delineate the normal range for blood APRIL mRNA. In four patients, blood APRIL mRNA levels were markedly raised despite normal serum APRIL levels (B, C). In most patients, blood APRIL mRNA levels remained normal throughout the entire observation periods (D, E).
Figure 5.
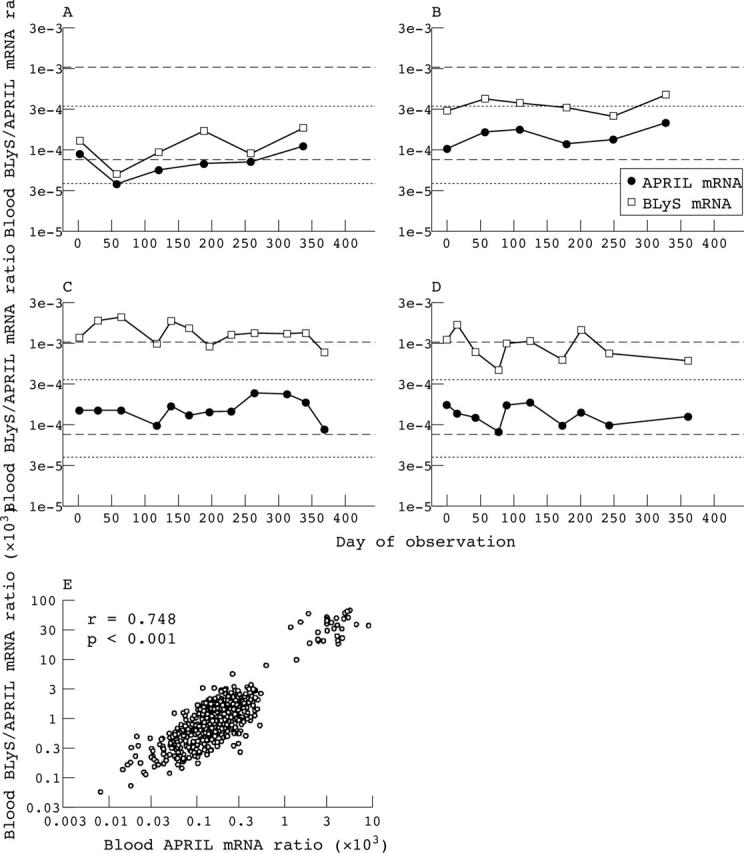
Relation between blood APRIL mRNA levels and blood BLyS mRNA levels. (A–D) Blood collected from two representative normal controls (A, B) and two representative patients with SLE (C, D) were assayed for APRIL mRNA and BLyS mRNA. The dotted lines delineate the normal range for blood APRIL mRNA, and the dashed lines delineate the normal range for blood BLyS mRNA.15 (E) Blood BLyS mRNA levels plotted against blood APRIL mRNA levels for the 526 individual blood samples obtained from the 68 patients with SLE for whom both blood APRIL and BLyS mRNA levels were determined.
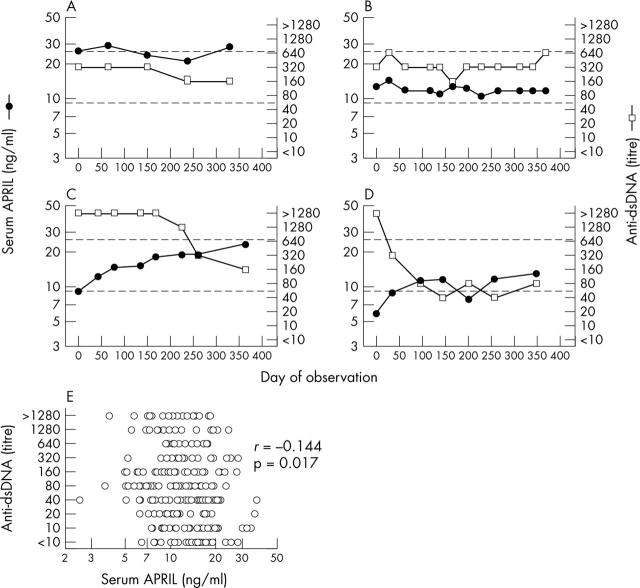
Figure 6.
Relation between serum APRIL levels and serum anti-dsDNA titres. (A, B) Serum APRIL levels (filled circles) and serum anti-dsDNA titres (open squares) were determined in two representative patients with SLE in whom changes in serum APRIL levels largely paralleled those in serum anti-dsDNA titres. (C, D) Serum APRIL levels and serum anti-dsDNA titres were determined in two representative patients with SLE in whom changes in serum APRIL levels were largely anti-parallel to those in serum anti-dsDNA titres. (E) Serum anti-dsDNA titres plotted against serum APRIL levels for the 275 individual serum samples obtained from the 34 anti-dsDNA positive patients with SLE.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery Danielle T., Kalled Susan L., Ellyard Julia I., Ambrose Christine, Bixler Sarah A., Thien Marilyn, Brink Robert, Mackay Fabienne, Hodgkin Philip D., Tangye Stuart G. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003 Jul;112(2):286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker Kevin P., Edwards Bryan M., Main Sarah H., Choi Gil H., Wager Ruth E., Halpern Wendy G., Lappin Patrick B., Riccobene Todd, Abramian Donara, Sekut Les. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003 Nov;48(11):3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- Batten M., Groom J., Cachero T. G., Qian F., Schneider P., Tschopp J., Browning J. L., Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000 Nov 20;192(10):1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Cheema G. S., Roschke V., Hilbert D. M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001 Jun;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Claudio Estefania, Brown Keith, Park Sun, Wang Hongshan, Siebenlist Ulrich. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002 Sep 23;3(10):958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- Craxton Andrew, Magaletti Dario, Ryan Elizabeth J., Clark Edward A. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003 Jan 16;101(11):4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- Do R. K., Hatada E., Lee H., Tourigny M. R., Hilbert D., Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000 Oct 2;192(7):953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. A., Johnston J., Mudri S., Enselman R., Dillon S. R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000 Apr 27;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- Hahne M., Kataoka T., Schröter M., Hofmann K., Irmler M., Bodmer J. L., Schneider P., Bornand T., Holler N., French L. E. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998 Sep 21;188(6):1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Benjamin L., Harless Susan M., Lindsley R. Coleman, Hilbert David M., Cancro Michael P. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002 Jun 15;168(12):5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- Kayagaki Nobuhiko, Yan Minhong, Seshasayee Dhaya, Wang Hua, Lee Wyne, French Dorothy M., Grewal Iqbal S., Cochran Andrea G., Gordon Nathaniel C., Yin JianPing. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002 Oct;17(4):515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- Khare S. D., Sarosi I., Xia X. Z., McCabe S., Miner K., Solovyev I., Hawkins N., Kelley M., Chang D., Van G. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Tsukamoto H., Masumoto K., Himeji D., Hayashi K., Harada M., Horiuchi T. A novel polymorphism of the human APRIL gene is associated with systemic lupus erythematosus. Rheumatology (Oxford) 2003 Mar 31;42(8):980–985. doi: 10.1093/rheumatology/keg270. [DOI] [PubMed] [Google Scholar]
- Litinskiy Mikhail B., Nardelli Bernardetta, Hilbert David M., He Bing, Schaffer Andras, Casali Paolo, Cerutti Andrea. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002 Aug 5;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Woodcock S. A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J. L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999 Dec 6;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsters S. A., Yan M., Pitti R. M., Haas P. E., Dixit V. M., Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000 Jun 29;10(13):785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- Moore P. A., Belvedere O., Orr A., Pieri K., LaFleur D. W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999 Jul 9;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Ni J., Zhai Y., Yu G. L., Aggarwal B. B. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999 Jun 4;274(23):15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- Nardelli B., Belvedere O., Roschke V., Moore P. A., Olsen H. S., Migone T. S., Sosnovtseva S., Carrell J. A., Feng P., Giri J. G. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001 Jan 1;97(1):198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- Pradet-Balade B., Medema J. P., López-Fraga M., Lozano J. C., Kolfschoten G. M., Picard A., Martínez-A C., Garcia-Sanz J. A., Hahne M. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO J. 2002 Nov 1;21(21):5711–5720. doi: 10.1093/emboj/cdf565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert P., Schneider P., Cachero T. G., Thompson J., Trabach L., Hertig S., Holler N., Qian F., Mullen C., Strauch K. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000 Dec 4;192(11):1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J. L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999 Jun 7;189(11):1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasayee Dhaya, Valdez Patricia, Yan Minhong, Dixit Vishva M., Tumas Daniel, Grewal Iqbal S. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003 Feb;18(2):279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- Shu H. B., Hu W. H., Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999 May;65(5):680–683. [PubMed] [Google Scholar]
- Stein Jens V., López-Fraga Marta, Elustondo Fernando A., Carvalho-Pinto Carla E., Rodríguez Dolores, Gómez-Caro Ruth, De Jong Joan, Martínez-A Carlos, Medema Jan Paul, Hahne Michael. APRIL modulates B and T cell immunity. J Clin Invest. 2002 Jun;109(12):1587–1598. doi: 10.1172/JCI15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl William, Metyas Samy, Tan Soon-Min, Cheema Gurtej S., Oamar Bonifacia, Xu Dong, Roschke Viktor, Wu Youmei, Baker Kevin P., Hilbert David M. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003 Dec;48(12):3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- Tan Soon-Min, Xu Dong, Roschke Viktor, Perry James W., Arkfeld Daniel G., Ehresmann Glenn R., Migone Thi-Sau, Hilbert David M., Stohl William. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003 Apr;48(4):982–992. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Bixler S. A., Qian F., Vora K., Scott M. L., Cachero T. G., Hession C., Schneider P., Sizing I. D., Mullen C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001 Aug 16;293(5537):2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Schneider P., Kalled S. L., Wang L., Lefevre E. A., Cachero T. G., MacKay F., Bixler S. A., Zafari M., Liu Z. Y. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000 Jul 3;192(1):129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribouley C., Wallroth M., Chan V., Paliard X., Fang E., Lamson G., Pot D., Escobedo J., Williams L. T. Characterization of a new member of the TNF family expressed on antigen presenting cells. Biol Chem. 1999 Dec;380(12):1443–1447. doi: 10.1515/BC.1999.186. [DOI] [PubMed] [Google Scholar]
- Wu Y., Bressette D., Carrell J. A., Kaufman T., Feng P., Taylor K., Gan Y., Cho Y. H., Garcia A. D., Gollatz E. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000 Nov 10;275(45):35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- Yan M., Brady J. R., Chan B., Lee W. P., Hsu B., Harless S., Cancro M., Grewal I. S., Dixit V. M. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001 Oct 2;11(19):1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- Yu G., Boone T., Delaney J., Hawkins N., Kelley M., Ramakrishnan M., McCabe S., Qiu W. R., Kornuc M., Xia X. Z. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000 Sep;1(3):252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- Zhang J., Roschke V., Baker K. P., Wang Z., Alarcón G. S., Fessler B. J., Bastian H., Kimberly R. P., Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001 Jan 1;166(1):6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]