Abstract
Background: The heteropolymer technology was developed to remove pathogens from the circulation.
Objectives: To evaluate the safety and tolerability of a single administration and to establish proof of principle for ETI-104 in normal healthy volunteers (NHV) and patients with systemic lupus erythematosus (SLE)
Methods: The drug was given intravenously to 11 NHV and six patients with SLE. Over 28 days, vital signs were noted, a haematological and chemical analysis of blood and urine was carried out, and adverse events were recorded. CR1 receptor numbers, the ability of antigen based heteropolymers to bind to red blood cells (RBCs), and the clearance of high avidity and total anti-dsDNA antibodies were measured by Farr assays and FACS analysis.
Results: No safety measure differed significantly from normal in both groups; no drug related serious adverse events occurred. ETI-104 rapidly bound to RBCs in NHV and patients with SLE. Binding of the drug to RBCs of patients with SLE also caused a rapid reduction of circulating anti-dsDNA antibodies in the plasma 15 minutes after administration, with a maximum reduction of 55% (range 43–62). At 28 days statistically significant decreases were maintained in three patients, while in the other three the values had returned to baseline levels.
Conclusion: These clinical trials established the safety and the proof of principle of the new immunoconjugate ETI-104. This provides the basis for further development of this technology for numerous indications—for example, therapeutic options for autoimmune diseases or viral and bacterial infections.
Full Text
The Full Text of this article is available as a PDF (195.1 KB).
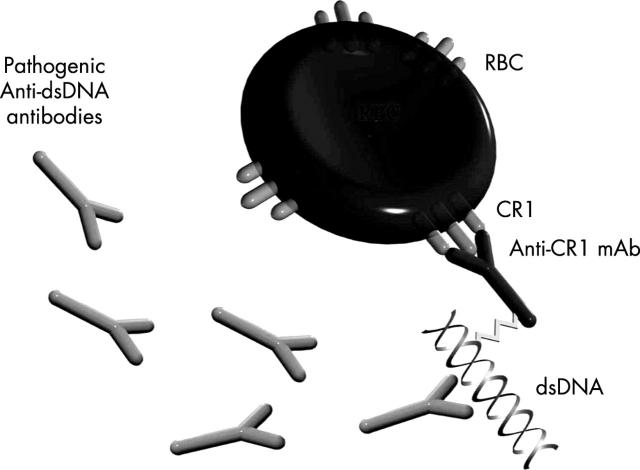
Figure 1.
Scheme of ETI-104 bound to an RBC.
Figure 2.
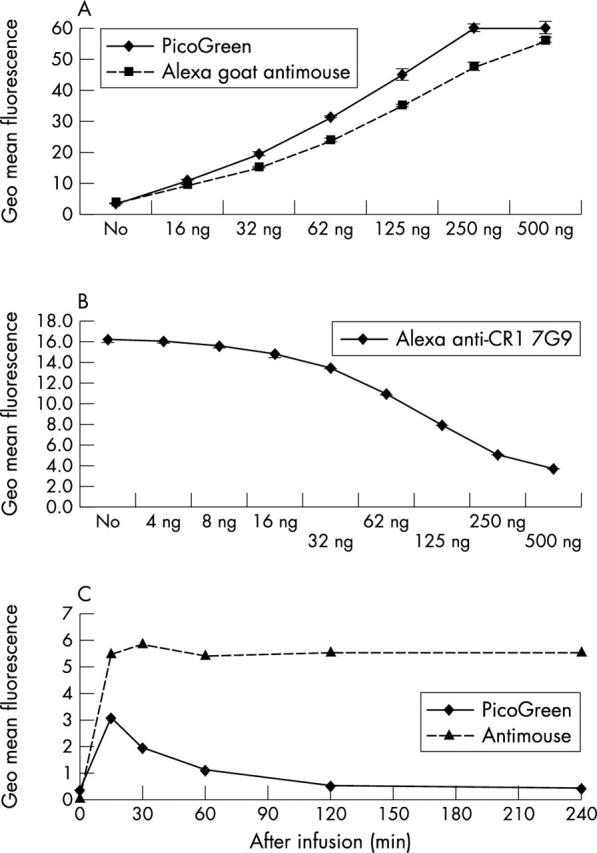
FACS analysis establishes binding of ETI-104 to RBCs of NHV. (A) Binding of ETI-104 to RBCs of each individual was confirmed by FACS analysis in vitro (goat antimouse for detection of the murine CR1 antibody and the PicoGreen DNA component of ETI-104). (B) RBCs from NHV 103 were bound in vitro to a saturating amount of Alexa-488 labelled anti-CR1 monoclonal antibody (mAb; 7G9) in the presence of varying concentrations of AHP. Increasing concentrations of AHP blocked 7G9 binding to the cells, demonstrating that AHP binds specifically to CR1. Thus, 250 ng AHP was not sufficient to block all available CR1. (C) RBCs from NHV 103 were stained after drug infusion to detect binding of the murine CR1 mAb (antimouse) and DNA (PicoGreen) components of ETI-104. The average of the geometric mean fluorescence intensity after subtracting non-specific fluorescence is given. The pattern of staining was similar in the other 10 NHV. All values were measured in duplicate.
Figure 3.
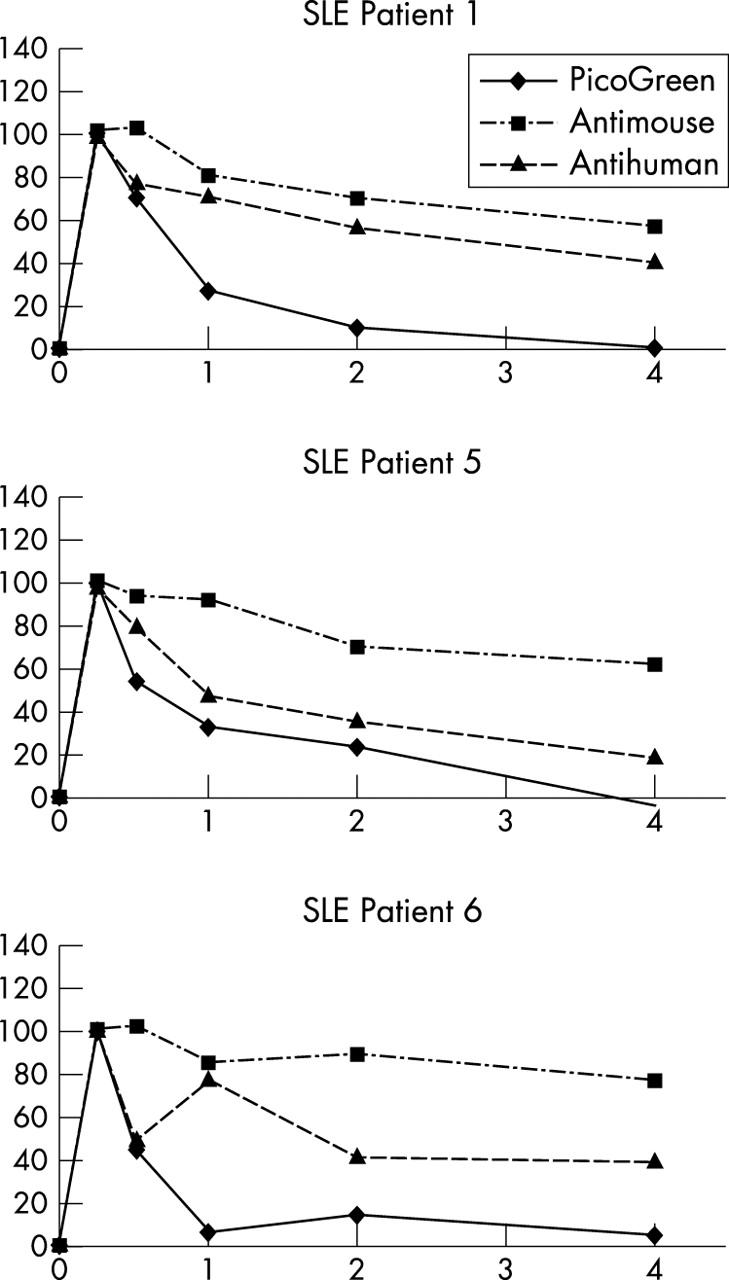
FACS analysis establishes binding of ETI-104 and target anti-dsDNA antibodies to RBCs in patients with SLE in vivo. RBCs from patients were stained after drug infusion to detect binding of the murine mAb (antimouse) and DNA (PicoGreen) components of ETI-104 and binding of the target anti-dsDNA antibody (antihuman). Average of the geometric mean fluorescence intensity of stained samples, after subtracting non-specific fluorescence, at each time was normalised to the 15 minute post-infusion value, which was set at 100%. The patients shown here represent the three patterns of association of anti-dsDNA antibodies with the RBCs; anti-dsDNA antibody tracked with the murine mAb component of ETI-104 (patient 1), with the DNA component of ETI-104 (patient 5), or non-parallel to either ETI-104 component (patient 6). Patients 2 and 3 showed essentially the same pattern as patient 1 and patient 4 as patient 5 (not shown). All values were measured in duplicate.
Figure 4.
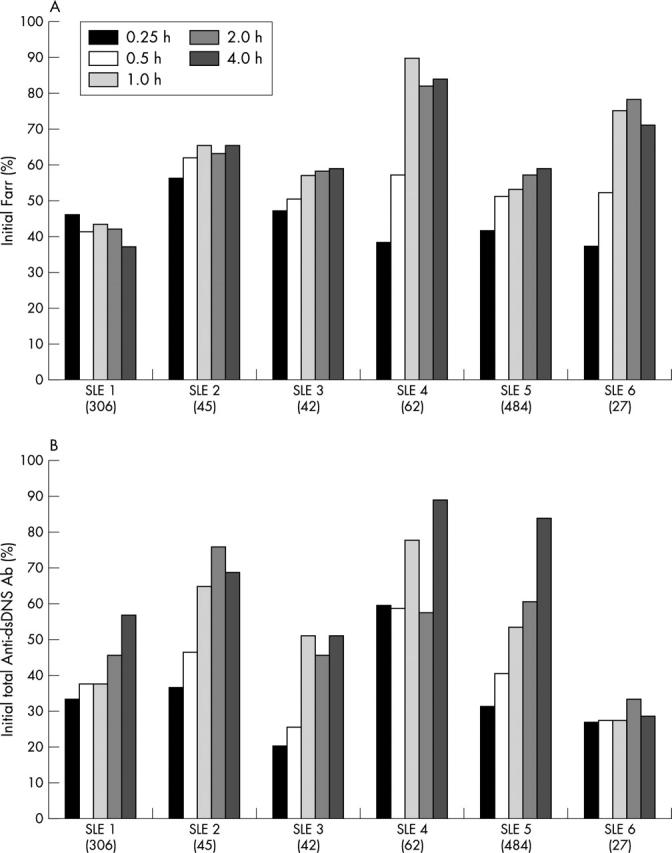
Administration of ETI-104 reduces the high avidity anti-dsDNA antibodies (Farr activity) and total dsDNA antibodies (FACS analysis) from the circulation of patients with SLE. (A) High avidity anti-dsDNA antibodies (Farr titre) reductions from 15 minutes to 4 hours after ETI-104 administration. The Farr titre was reduced from the initial values by 43–62% at 15 minutes and by 15–62% at 4 hours after ETI-104 administration; it was maximally reduced in patient No 1 at this time. The reduction was ∼200 IU/ml in patients with starting titres of 306 and 484 IU/ml (starting titres of each patient are given in brackets). All Farr values were measured in quadruplicate. (B) Total anti-dsDNA antibody (FACS analysis) reductions from 15 minutes to 4 hours after ETI-104 administration. Patient plasma samples were incubated in vitro with naïve AHP opsonised RBCs (blood group O), washed, and stained with Alexa antihuman IgG and IgM antibody. Total dsDNA antibody levels were reduced from their initial value by 40–79% at 15 minutes and by 11–71% at 4 hours after ETI-104 administration. All FACS values were measured in duplicate.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., Lakmaker F., De Groot E. R. Immunology of DNA. IV. Quantitative aspects of the Farr assay. J Immunol Methods. 1976;11(2):153–163. doi: 10.1016/0022-1759(76)90143-5. [DOI] [PubMed] [Google Scholar]
- Alarcón-Segovia Donato, Tumlin James A., Furie Richard A., McKay James D., Cardiel Mario H., Strand Vibeke, Bagin Robert G., Linnik Matthew D., Hepburn Bonnie, LJP 394 Investigator Consortium LJP 394 for the prevention of renal flare in patients with systemic lupus erythematosus: results from a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2003 Feb;48(2):442–454. doi: 10.1002/art.10763. [DOI] [PubMed] [Google Scholar]
- Bootsma H., Spronk P. E., Ter Borg E. J., Hummel E. J., de Boer G., Limburg P. C., Kallenberg C. G. The predictive value of fluctuations in IgM and IgG class anti-dsDNA antibodies for relapses in systemic lupus erythematosus. A prospective long-term observation. Ann Rheum Dis. 1997 Nov;56(11):661–666. doi: 10.1136/ard.56.11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacoff J. B., Hebert L. A., Smead W. L., VanAman M. E., Birmingham D. J., Waxman F. J. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983 Feb;71(2):236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Emlen W., Jarusiripipat P., Burdick G. A new ELISA for the detection of double-stranded DNA antibodies. J Immunol Methods. 1990 Aug 28;132(1):91–101. doi: 10.1016/0022-1759(90)90402-h. [DOI] [PubMed] [Google Scholar]
- Emlen W., Pisetsky D. S., Taylor R. P. Antibodies to DNA. A perspective. Arthritis Rheum. 1986 Dec;29(12):1417–1426. doi: 10.1002/art.1780291201. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson P. J., Reist C. J., Martin E. N., Johnson C., Greene K. L., Kuhn S., Niebur J., Emlen W., Taylor R. P. Antigen-based heteropolymers. A potential therapy for binding and clearing autoantibodies via erythrocyte CR1. Arthritis Rheum. 1995 Feb;38(2):190–200. doi: 10.1002/art.1780380207. [DOI] [PubMed] [Google Scholar]
- Gilkeson G. S., Bernstein K., Pippen A. M., Clarke S. H., Marion T., Pisetsky D. S., Ruiz P., Lefkowith J. B. The influence of variable-region somatic mutations on the specificity and pathogenicity of murine monoclonal anti-DNA antibodies. Clin Immunol Immunopathol. 1995 Jul;76(1 Pt 1):59–67. doi: 10.1006/clin.1995.1088. [DOI] [PubMed] [Google Scholar]
- Hebert L. A., Cosio G. The erythrocyte-immune complex-glomerulonephritis connection in man. Kidney Int. 1987 Apr;31(4):877–885. doi: 10.1038/ki.1987.81. [DOI] [PubMed] [Google Scholar]
- Hecht B., Siegel N., Adler M., Kashgarian M., Hayslett J. P. Prognostic indices in lupus nephritis. Medicine (Baltimore) 1976 Mar;55(2):163–181. doi: 10.1097/00005792-197603000-00005. [DOI] [PubMed] [Google Scholar]
- Ho A., Magder L. S., Barr S. G., Petri M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001 Oct;44(10):2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jouvin M. H., Wilson J. G., Bourgeois P., Fearon D. T., Kazatchkine M. D. Decreased expression of C3b receptor (CR1) on erythrocytes of patients with systemic lupus erythematosus contrasts with its normal expression in other systemic diseases and does not correlate with the occurrence or severity of SLE nephritis. Complement. 1986;3(2):88–96. doi: 10.1159/000467884. [DOI] [PubMed] [Google Scholar]
- Koffler D., Agnello V., Kimkel H. G. Polynucleotide immune complexes in serum and glomeruli of patients with systemic lupus erythematosus. Am J Pathol. 1974 Jan;74(1):109–124. [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C., Kaplan M. H. Immunopathologic studies of systemic lupus erythematosus. II. Antinuclear reaction of gamma-globulin eluted from homogenates and isolated glomeruli of kidneys from patients with lupus nephritis. J Clin Invest. 1967 Apr;46(4):569–579. doi: 10.1172/JCI105558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindorfer M. A., Hahn C. S., Foley P. L., Taylor R. P. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol Rev. 2001 Oct;183:10–24. doi: 10.1034/j.1600-065x.2001.1830102.x. [DOI] [PubMed] [Google Scholar]
- Lindorfer M. A., Schuman T. A., Craig M. L., Martin E. N., Taylor R. P. A bispecific dsDNAxmonoclonal antibody construct for clearance of anti-dsDNA IgG in systemic lupus erythematosus. J Immunol Methods. 2001 Feb 1;248(1-2):125–138. doi: 10.1016/s0022-1759(00)00348-3. [DOI] [PubMed] [Google Scholar]
- McNeeley P. A., Iverson G. M., Furie R. A., Cash J. M., Cronin M. E., Katz R. S., Weisman M. H., Aranow C., Linnik M. D. Pre-treatment affinity for LJP 394 influences pharmacodynamic response in lupus patients. Lupus. 2001;10(8):526–532. doi: 10.1191/096120301701549642. [DOI] [PubMed] [Google Scholar]
- Miniter M. F., Stollar B. D., Agnello V. Reassessment of the clinical significance of native DNA antibodies in systemic lupus erythematosus. Arthritis Rheum. 1979 Sep;22(9):959–968. doi: 10.1002/art.1780220903. [DOI] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; a hypothetical role of erythrocytes in defence against bacteria and viruses. Proc R Soc Med. 1956 Jan;49(1):55–58. [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- Petri M. Hopkins Lupus Cohort. 1999 update. Rheum Dis Clin North Am. 2000 May;26(2):199-213, v. doi: 10.1016/s0889-857x(05)70135-6. [DOI] [PubMed] [Google Scholar]
- Pincus Steven E., Lukacher Nadya, Mohamed Nehal, Sesay Muctarr, Zabinski Roger, Ebelle Rose, Duncan Lisa, Li Juan, Chen Xun, Peng Wu. Evaluation of antigen-based heteropolymer for treatment of systemic lupus erythematosus in a nonhuman primate model. Clin Immunol. 2002 Nov;105(2):141–154. doi: 10.1006/clim.2002.5274. [DOI] [PubMed] [Google Scholar]
- Schifferli J. A., Taylor R. P. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989 Apr;35(4):993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- Spertini F., Leimgruber A., Morel B., Khazaeli M. B., Yamamoto K., Dayer J. M., Weisbart R. H., Lee M. L. Idiotypic vaccination with a murine anti-dsDNA antibody: phase I study in patients with nonactive systemic lupus erythematosus with nephritis. J Rheumatol. 1999 Dec;26(12):2602–2608. [PubMed] [Google Scholar]
- Swaak A. J., Aarden L. A., Statius van Eps L. W., Feltkamp T. E. Anti-dsDNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum. 1979 Mar;22(3):226–235. doi: 10.1002/art.1780220304. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Schur P. H., Carr R. I., Kunkel H. G. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966 Nov;45(11):1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. P., Sutherland W. M., Martin E. N., Ferguson P. J., Reinagel M. L., Gilbert E., Lopez K., Incardona N. L., Ochs H. D. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol. 1997 Jan 15;158(2):842–850. [PubMed] [Google Scholar]
- Tsao B. P., Ohnishi K., Cheroutre H., Mitchell B., Teitell M., Mixter P., Kronenberg M., Hahn B. H. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J Immunol. 1992 Jul 1;149(1):350–358. [PubMed] [Google Scholar]
- Vlahakos D. V., Foster M. H., Adams S., Katz M., Ucci A. A., Barrett K. J., Datta S. K., Madaio M. P. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992 Jun;41(6):1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- Wallace Daniel J. Management of lupus erythematosus: recent insights. Curr Opin Rheumatol. 2002 May;14(3):212–219. doi: 10.1097/00002281-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Gharavi A. E., Koike T., Lockshin M. D., Branch D. W., Piette J. C., Brey R., Derksen R., Harris E. N., Hughes G. R. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999 Jul;42(7):1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Faiferman I., Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977 Jan;59(1):90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J. H., Liu H. W., Lin S. F., Chen J. R., Chen T. P. Erythrocyte complement receptor type 1 in patients with systemic lupus erythematosus. J Rheumatol. 1989 Oct;16(10):1320–1325. [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E. J., Limburg P. C., Kallenberg C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990 May;33(5):634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]