Abstract
Objectives: To improve the understanding of epicondylitis by describing the normal structure and composition of the entheses associated with the medial and lateral epicondyles and their histopathology in elderly cadavers.
Methods: Medial and lateral epicondyles were obtained from 12 cadavers. Six middle aged cadavers (mean 47 years) were used to assess the molecular composition of "normal" entheses from people within an age range vulnerable to epicondylitis. Cryosections of epicondylar entheses were immunolabelled with monoclonal antibodies against molecules associated with fibrocartilage and related tissues. A further six elderly cadavers (mean 84 years) were used for histology to assess features of entheses related to increasing age.
Results: Tendon entheses on both epicondyles fused with those of the collateral ligaments and formed a more extensive structure than hitherto appreciated. Fibrocartilage (which labelled for type II collagen and aggrecan) was a constant feature of all entheses. Entheses from elderly subjects showed extensive microscopic damage, hitherto regarded as a hallmark of epicondylitis.
Conclusions: Fibrocartilage is a normal feature and not always a sign of enthesopathy. Furthermore, pathological changes documented in patients with epicondylitis may also be seen in elderly people. The fusion of the common extensor and flexor tendon entheses with those of the collateral ligaments suggests that the latter may be implicated as well. This may explain why pain and tenderness in epicondylitis may extend locally beyond the tendon enthesis and why some patients are refractory to local treatments.
Full Text
The Full Text of this article is available as a PDF (635.2 KB).
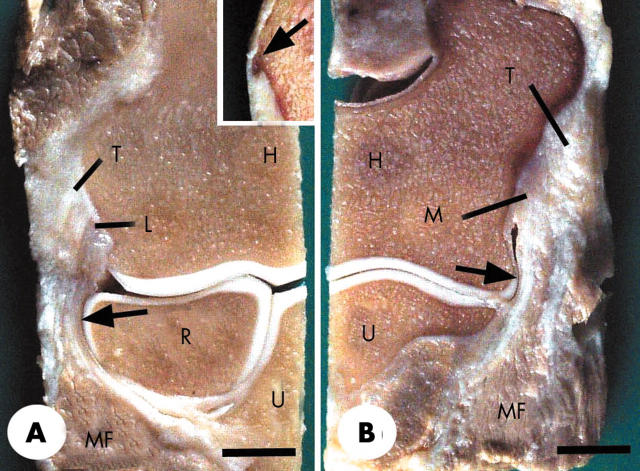
Figure 1.
Frontal longitudinal sections of a normal elbow joint from a 30 year old man. (A) The radial (lateral) part of the joint showing the tendinous attachment (T) of the common extensor origin to the lateral epicondyle and the attachment of the lateral collateral ligament (L) with which it was directly continuous. Note that the collateral ligament merges with the annular ligament (arrow), which in turn wraps around the head of the radius (R). Note also the presence of muscle fibres (MF) attaching to both the tendon and the collateral ligament. H, humerus; U, ulna. Inset—the lateral epicondyle from a 47 year old man with a small bony spur (arrow) at the tendon enthesis. (B) The ulnar (medial) part of the same specimen that is featured in (A). The tendinous attachment (T) of the common flexor origin is directly continuous with the medial collateral ligament (M). Note that the ligament wraps around the articular cartilage at the edge of the trochlea (arrow) at the lower end of the humerus (H) and that again there are muscle fibres (MF) attaching to both the tendon and the medial collateral ligament. U, ulna; Scale bars = 0.9 cm.
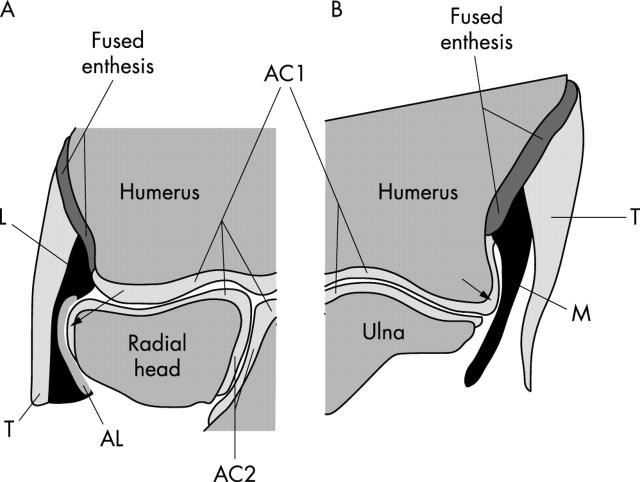
Figure 2.
A schematic representation of the enthesis organs associated with the medial and lateral epicondyles based on fig 1. The elbow is thus drawn in coronal (frontal) section with the humerus located proximally and the radius and ulna distally. Both the articular cartilages of the elbow (AC1) and superior radioulnar (AC2) joints are visible. (A) The lateral epicondyle. The lateral collateral ligament (L) fuses with the annular ligament (AL), which in turn wraps around the radial head at the position of the arrow. The common extensor tendon (T) lies immediately lateral to the collateral ligament and the entheses of these two structures are fused. The single fused enthesis serves to dissipate stress concentration between tendon and ligament at the bony interface and stress is further reduced by the contact between annular ligament and radial head (arrow). Consequently, the enthesis organ comprises the tendon, collateral ligament, annular ligament, and the adjacent circumference of the radial head. (B) The medial epicondyle. The medial collateral ligament (M) fuses with the tendon (T) of the common flexor origin at its enthesis and the former presses against the articular cartilage on the edge of the humerus at the position of the arrow. The enthesis organ thus consists of the tendon, collateral ligament, and a part of the humeral articular cartilage.
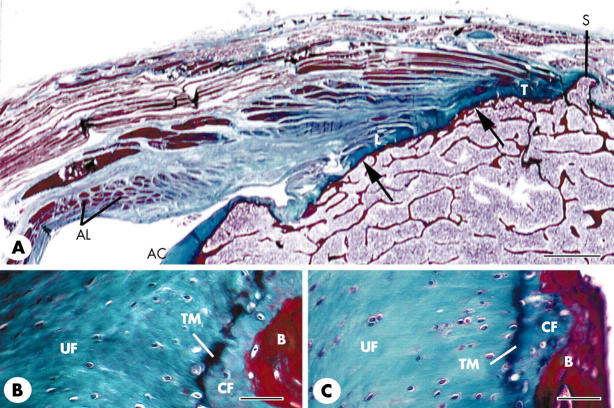
Figure 3.
Routine histology and histopathology of the medial and lateral epicondylar entheses. (A) A low power view (frontal section) of the single enthesis on the lateral epicondyle from a 101 year old woman that is formed by the fusion of the tendon (T) of the common extensor origin with the lateral collateral ligament (L) of the elbow joint. The tendon and collateral ligament similarly fuse with each other at the medial epicondylar enthesis (not illustrated). Note that the lateral collateral ligament is also fused with the annular ligament (AL) that wraps around around the head of the radius (not visible in the section). Note also the presence of only a thin layer of subchondral bone (arrows) throughout the enthesis and the small bony spur (S) at the proximal end of the tendon attachment. AC, articular cartilage. Scale bar = 0.2 cm. (B, C) Fibrocartilage at the attachment of both the tendon (B) and the ligament (C) to the lateral epicondyle in the same specimen featured in (A). The fibrocartilage is equally prominent at both sites and consists of zones of uncalcified (UF) and calcified (CF) tissue separated by a calcification front known as the tidemark (TM). B, bone. Scale bars = 50 µm.
Figure 4.
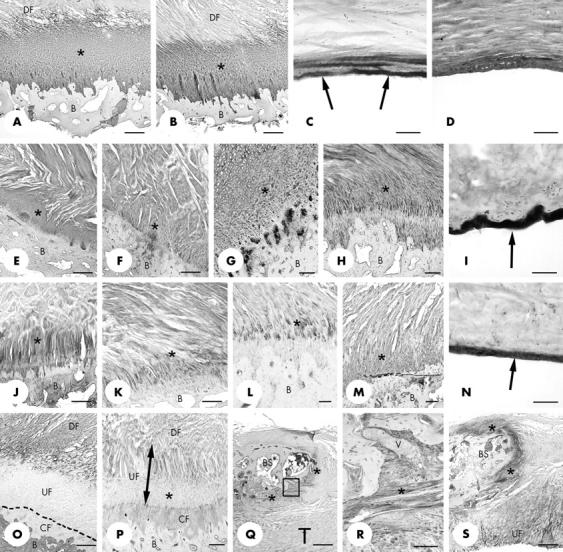
Immunohistochemical labelling of the medial and lateral epicondylar entheses. (A, B) Extensive labelling of fibrocartilage for type II collagen (asterisk) at both the tendon (A) and ligament (B) parts of the lateral epicondylar enthesis. B, bone; DF dense fibrous connective tissue. Scale bars = 500 µm. (C) Strong labelling for type II collagen (arrows) in the region of the medial collateral ligament close to the enthesis, where it wraps around the edge of the humeral articular cartilage. The region illustrated is that indicated by the arrow in fig 1B. Note that only the surface of the ligament is labelled. Scale bar = 100 µm. (D) At the lateral epicondylar enthesis, labelling of the "wrap-around" region of the lateral collateral ligament (that is, where it was in contact with the head of the radius—see arrow in fig 1A) for type II collagen was seen not only at the ligament surface but also in the underlying tissue. Scale bar = 100 µm. (E, F) Aggrecan labelling (asterisk) of fibrocartilage at the ligament (E) and tendon (F) parts of the medial epicondylar enthesis. B, bone. Scale bars = 500 µm. (G, H) Aggrecan labelling (asterisk) of fibrocartilage at the ligament (G) and tendon (H) parts of the lateral epicondylar enthesis. B, bone. Scale bars = 250 µm. (I) Intense labelling for aggrecan at the surface (arrow) of the "wrap-around" region of the lateral collateral ligament (that is, where it was in contact with the head of the radius—see arrow in fig 1A). Scale bar = 50 µm. (J, K) Labelling for link protein (asterisk) in fibrocartilage at both the ligament (J) and tendon (K) parts of the lateral epicondylar enthesis. B, bone. Scale bars = 500 µm. (L) Chondroitin-6-sulphate labelling (asterisk) in fibrocartilage in the tendon part of the medial epicondylar enthesis. B, bone. Scale bar = 100 µm. (M) Chondroitin-6-sulphate labelling (asterisk) in fibrocartilage in the ligament part of the lateral epicondylar enthesis. B, bone. Scale bar = 100 µm. (N) Strong labelling for chondroitin-6-sulphate (arrow) in the region of the lateral collateral ligament close to the enthesis, where it wraps around the head of the radius. The region illustrated is that indicated by the arrow in fig 1A. Scale bar = 50 µm. (O) Type I collagen labelling in the ligament part of the lateral epicondylar enthesis. Note the strong labelling of both the dense fibrous zone of the ligament enthesis (DF) and the bone (B), but the absence of labelling from the calcified (CF) and much of the uncalcified enthesis fibrocartilage (UF). The dotted line indicates the position of the tidemark. Scale bar = 500 µm. (P) Versican labelling in the ligament part of the lateral epicondylar enthesis. Labelling is present in the dense fibrous zone (DF), part of the adjacent zone of uncalcified fibrocartilage (UF), and the zone of calcified fibrocartilage (CF). However, labelling was largely absent from the deeper part of the zone of uncalcified fibrocartilage (asterisk). B, bone. Scale bar = 250 µm. (Q) Low power view of type II collagen labelling (asterisk) of fibrocartilage at the surface of a small bony spur (BS) in the tendon (T) part of the lateral epicondylar enthesis. Scale bar = 500 µm. (R) High power view of the region enclosed in the rectangle in (Q) showing a small venule (V) packed with blood cells, passing between tendon and bone in the type II collagen positive region (asterisk). Scale bar = 250 µm. (S) Strong labelling for link protein (asterisk) around the tip of the bony spur (BS) featured in (Q) and (R) and in the uncalcified fibrocartilage (UF) of the tendon. A similar labelling pattern was also evident for aggrecan. Scale bar = 500 µm.
Figure 5.
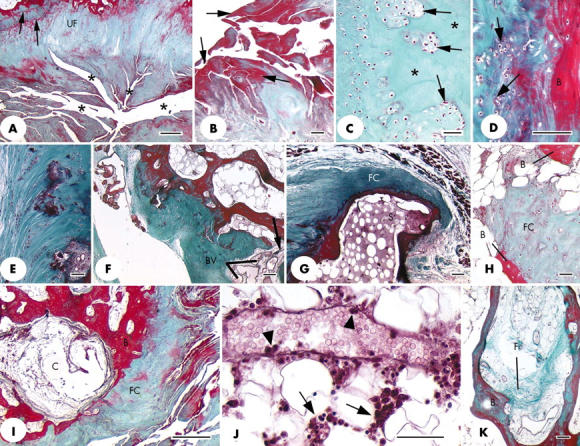
(A) Prominent fissures (*) at the ligament end of the medial epicondylar enthesis that greatly disrupt the normal structure of the attachment site. UF, uncalcified fibrocartilage; arrows, tidemarks. Scale bar = 500 µm. (B) Higher power view of several fissures from the same specimen as illustrated in (A) to show the prominent layer of fibrin (arrows) on the surface of the fissures. Scale bar = 50 µm. (C) Clusters of fibrocartilage cells (arrows) separated by small regions of acellular matrix (asterisk) in the tendon part of a lateral epicondylar enthesis. Scale bar = 50 µm. (D) Fibrocartilage cell proliferation (arrows) at the tendon attachment on the lateral epicondylar enthesis. B, bone. Scale bar = 100 µm. (E) Small foci of calcified tissue and fibrocartilage cell clusters in the zone of uncalcified fibrocartilage at the medial epicondylar enthesis. Scale bar = 50 µm. (F) Numerous small blood vessels (BV), indicating vascular proliferation at the site of a small bony defect (arrow) in the tendon attachment on the medial epicondylar enthesis. The blood vessels are passing between the tendon and the bone marrow. Scale bar = 100 µm. (G) A section adjacent to that featured in fig 2A to show the presence of fibrocartilage (FC) at the tip of the small bony spur (S). (H) A small region of fibrocartilage (FC) within the bone marrow, between neighbouring bone spicules (B). Scale bar = 50 µm. (I) A subchondral bone cyst (C) beneath the tendinous part of the lateral epicondylar enthesis. B, bone; FC, fibrocartilage. Scale bar = 500 µm. (J) A venule in the bone marrow associated with the tendinous part of the lateral epicondylar enthesis. Note the presence of inflammatory cells around the vessel (arrows) and of several marginating leucocytes around the edge of the lumen (arrowheads). Scale bar = 50 µm. (K) Evidence of bone marrow fibrosis (F) in the same specimen as (J). B, bone. Scale bar = 100 µm. All sections were stained with Masson's trichrome.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin M., Evans E. J., Copp L. The histology of tendon attachments to bone in man. J Anat. 1986 Dec;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Kumai T., Milz S., Boszczyk B. M., Boszczyk A. A., Ralphs J. R. The skeletal attachment of tendons--tendon "entheses". Comp Biochem Physiol A Mol Integr Physiol. 2002 Dec;133(4):931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Benjamin M., McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001 Nov;199(Pt 5):503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. B. Lateral and medial epicondylitis. Hand Clin. 1994 Feb;10(1):157–163. [PubMed] [Google Scholar]
- Boszczyk Alexandra A., Boszczyk Bronek M., Putz Reinhard, Benjamin Michael, Milz Stefan. Expression of a wide range of fibrocartilage molecules at the entheses of the alar ligaments - possible antigenic targets for rheumatoid arthritis? J Rheumatol. 2003 Jul;30(7):1420–1425. [PubMed] [Google Scholar]
- Boszczyk B. M., Boszczyk A. A., Putz R., Büttner A., Benjamin M., Milz S. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine (Phila Pa 1976) 2001 Aug 1;26(15):E338–E343. doi: 10.1097/00007632-200108010-00006. [DOI] [PubMed] [Google Scholar]
- Boszczyk Bronek M., Boszczyk Alexandra A., Korge Andreas, Grillhösl Andreas, Boos Wolf-Dietrich, Putz Reinhard, Milz Stefan, Benjamin Michael. Immunohistochemical analysis of the extracellular matrix in the posterior capsule of the zygapophysial joints in patients with degenerative L4-5 motion segment instability. J Neurosurg. 2003 Jul;99(1 Suppl):27–33. doi: 10.3171/spi.2003.99.1.0027. [DOI] [PubMed] [Google Scholar]
- Braun J., Khan M. A., Sieper J. Enthesitis and ankylosis in spondyloarthropathy: what is the target of the immune response? Ann Rheum Dis. 2000 Dec;59(12):985–994. doi: 10.1136/ard.59.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B., Flannery C. R., Hughes C. E., Little C. B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000 Aug;19(4):333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Chard M. D., Cawston T. E., Riley G. P., Gresham G. A., Hazleman B. L. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994 Jan;53(1):30–34. doi: 10.1136/ard.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell D., Burke F., Coombes P., McNealy S., Freeman D., Pryde D., Hoy G. Sonographic examination of lateral epicondylitis. AJR Am J Roentgenol. 2001 Mar;176(3):777–782. doi: 10.2214/ajr.176.3.1760777. [DOI] [PubMed] [Google Scholar]
- Das D., Maffulli N. Surgical management of tennis elbow. J Sports Med Phys Fitness. 2002 Jun;42(2):190–197. [PubMed] [Google Scholar]
- Field L. D., Savoie F. H. Common elbow injuries in sport. Sports Med. 1998 Sep;26(3):193–205. doi: 10.2165/00007256-199826030-00005. [DOI] [PubMed] [Google Scholar]
- Galliani I., Burattini S., Mariani A. R., Riccio M., Cassiani G., Falcieri E. Morpho-functional changes in human tendon tissue. Eur J Histochem. 2002;46(1):3–12. doi: 10.4081/1649. [DOI] [PubMed] [Google Scholar]
- Galliani I., Columbaro M., Ferri S., Valmori A., Cassiani G., Maltarello M. C., Falcieri E. Calcific chronic lateral epicondylitis: a histological and ultrastructural study. J Submicrosc Cytol Pathol. 1997 Oct;29(4):453–459. [PubMed] [Google Scholar]
- Grundberg A. B., Dobson J. F. Percutaneous release of the common extensor origin for tennis elbow. Clin Orthop Relat Res. 2000 Jul;(376):137–140. doi: 10.1097/00003086-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Guerassimov A., Duffy C., Zhang Y., Banerjee S., Leroux J. Y., Reimann A., Webber C., Delaunay N., Vipparti V., Ronbeck L. Immunity to cartilage link protein in patients with juvenile rheumatoid arthritis. J Rheumatol. 1997 May;24(5):959–964. [PubMed] [Google Scholar]
- Guerassimov A., Zhang Y., Banerjee S., Cartman A., Webber C., Esdaile J., Fitzcharles M. A., Poole A. R. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998 Aug;25(8):1480–1484. [PubMed] [Google Scholar]
- Kandemir Utku, Fu Freddie H., McMahon Patrick J. Elbow injuries. Curr Opin Rheumatol. 2002 Mar;14(2):160–167. doi: 10.1097/00002281-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Kumagai J., Sarkar K., Uhthoff H. K. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994 Nov;21(11):2096–2100. [PubMed] [Google Scholar]
- Lehtinen J. T., Kaarela K., Ikävalko M., Kauppi M. J., Belt E. A., Kuusela P. P., Kautiainen H. J., Lehto M. U. Incidence of elbow involvement in rheumatoid arthritis. A 15 year endpoint study. J Rheumatol. 2001 Jan;28(1):70–74. [PubMed] [Google Scholar]
- Maksymowych W. P. Ankylosing spondylitis--at the interface of bone and cartilage. J Rheumatol. 2000 Oct;27(10):2295–2301. [PubMed] [Google Scholar]
- Milz S., Schlüter T., Putz R., Moriggl B., Ralphs J. R., Benjamin M. Fibrocartilage in the transverse ligament of the human atlas. Spine (Phila Pa 1976) 2001 Aug 15;26(16):1765–1771. doi: 10.1097/00007632-200108150-00007. [DOI] [PubMed] [Google Scholar]
- Milz S., Valassis G., Büttner A., Maier M., Putz R., Ralphs J. R., Benjamin M. Fibrocartilage in the transverse ligament of the human acetabulum. J Anat. 2001 Feb;198(Pt 2):223–228. doi: 10.1046/j.1469-7580.2001.19820223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl B., Jax P., Milz S., Büttner A., Benjamin M. Fibrocartilage at the entheses of the suprascapular (superior transverse scapular) ligament of man--a ligament spanning two regions of a single bone. J Anat. 2001 Nov;199(Pt 5):539–545. doi: 10.1046/j.1469-7580.2001.19950539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirschl R. P. Elbow tendinosis/tennis elbow. Clin Sports Med. 1992 Oct;11(4):851–870. [PubMed] [Google Scholar]
- Plancher K. D., Halbrecht J., Lourie G. M. Medial and lateral epicondylitis in the athlete. Clin Sports Med. 1996 Apr;15(2):283–305. [PubMed] [Google Scholar]
- Potter H. G., Hannafin J. A., Morwessel R. M., DiCarlo E. F., O'Brien S. J., Altchek D. W. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology. 1995 Jul;196(1):43–46. doi: 10.1148/radiology.196.1.7784585. [DOI] [PubMed] [Google Scholar]
- Putz R., Milz S., Maier M., Boszczyk A. Funktionelle Morphologie des Ellenbogengelenks. Orthopade. 2003 Aug;32(8):684–690. doi: 10.1007/s00132-003-0508-0. [DOI] [PubMed] [Google Scholar]
- Putz R., Reichelt A. Strukturelle Befunde am Lig. coracoacromiale bei Rotatorenmanschettenruptur, Tendinosis calcarea und Supraspinatussyndrom. Z Orthop Ihre Grenzgeb. 1990 Jan-Feb;128(1):46–50. doi: 10.1055/s-2008-1039860. [DOI] [PubMed] [Google Scholar]
- Regan W., Wold L. E., Coonrad R., Morrey B. F. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med. 1992 Nov-Dec;20(6):746–749. doi: 10.1177/036354659202000618. [DOI] [PubMed] [Google Scholar]
- Rufai A., Ralphs J. R., Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. 1995 Jul;13(4):585–593. doi: 10.1002/jor.1100130414. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER H. Zur Struktur der Sehnenansatzzonen. Z Anat Entwicklungsgesch. 1956;119(5):431–456. [PubMed] [Google Scholar]
- Steinborn M., Heuck A., Jessel C., Bonel H., Reiser M. Magnetic resonance imaging of lateral epicondylitis of the elbow with a 0.2-T dedicated system. Eur Radiol. 1999;9(7):1376–1380. doi: 10.1007/s003300050851. [DOI] [PubMed] [Google Scholar]
- Yahia H., Drouin G., Maurais G., Garzon S., Rivard C. H. Degeneration of the human lumbar spine ligaments. An ultrastructural study. Pathol Res Pract. 1989 Apr;184(4):369–375. doi: 10.1016/S0344-0338(89)80031-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guerassimov A., Leroux J. Y., Cartman A., Webber C., Lalic R., de Miguel E., Rosenberg L. C., Poole A. R. Induction of arthritis in BALB/c mice by cartilage link protein: involvement of distinct regions recognized by T and B lymphocytes. Am J Pathol. 1998 Oct;153(4):1283–1291. doi: 10.1016/S0002-9440(10)65673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]