Abstract
Background: T cells have a pivotal role in RA. Leflunomide inhibits pyrimidine biosynthesis, to which T cells are especially susceptible, and therefore may have a different cytokine profile than methotrexate.
Materials and methods: Serum samples of 100 patients with RA, treated with leflunomide (n = 50) or methotrexate (n = 50), were collected at baseline, after 16 weeks and after 1 year's treatment. Serum levels of interleukin 6 (IL6), and interferon (IFN) γ were determined by ELISA. Additionally, peripheral blood mononuclear cells (PBMC) of five healthy volunteers and three patients with RA were isolated and the effects of the active metabolite of leflunomide (A77-1726, 0–200 mmol/l) on cell proliferation and on IL6 and IFNγ production were determined by ELISA. In peripheral blood lymphocytes (PBL) and monocytes (PBM) from two healthy volunteers the effects of A77-1726 on IL6 production were measured by ELISA and PCR.
Results: Mean (SEM) serum levels of IFNγ were significantly reduced after leflunomide treatment (baseline 43 (10) pg/ml; 1 year 29 (7) (p = 0.015), but there was no change in IL6 levels (baseline 158 (41), 1 year 151 (48)). Both IFNγ and IL6 levels were significantly reduced after methotrexate treatment. This observation was supported by in vitro experiments. The production of IFNγ by PBL was inhibited by A77-1726, but IL6 production by PBM was not inhibited.
Conclusion: The differential effect on IFNγ and IL6 production supports the hypothesis that activated T cells are preferentially inhibited by leflunomide. An explanation may be either inhibition of uridine synthesis or effects on signal transduction pathways.
Full Text
The Full Text of this article is available as a PDF (166.2 KB).
Figure 1.
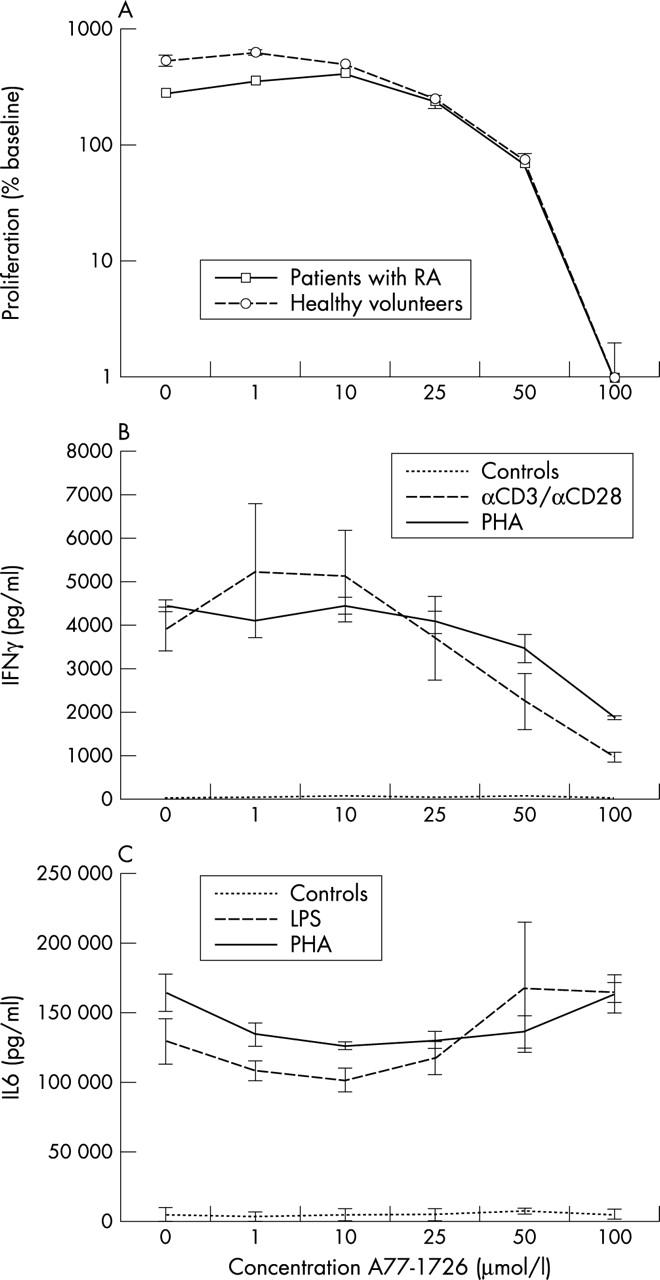
(A) Incorporation of [3H]thymidine by PBMC of healthy donors and patients with RA after stimulation with LPS. Graph depicts the number of counts as measured after 24 hours of incubation in the presence of various concentrations of leflunomide (0–100 µmol/l). (B) Production of IFNγ (pg/ml) by PBMC after 36 hours. Depicted are controls and cells stimulated with PHA in the presence of various concentrations of leflunomide (0–100 µmol/l). (C) Production of IL6 (pg/ml) by PBMC after 8 hours. Depicted are controls and cells stimulated with PHA in the presence of various concentrations of leflunomide (0–100 µmol/l).
Figure 2.
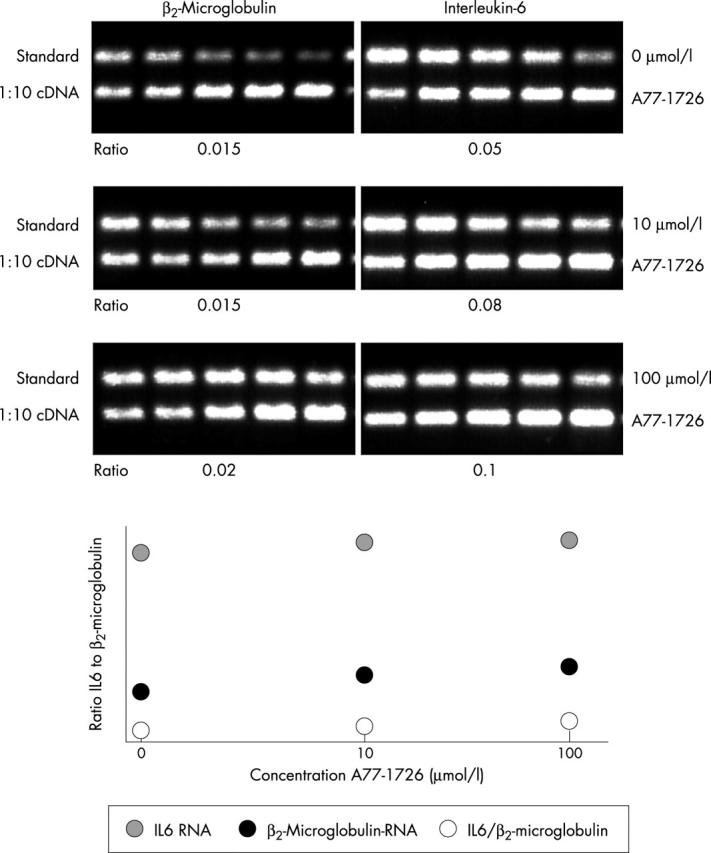
Analysis of ß2-microglobulin and IL6 mRNA levels in PHA stimulated monocytes. A constant volume of the ß2-microglobulin and IL6 cellular cDNA products was mixed with graded amounts of a known concentration of pQA1 DNA (st-DNA) containing the specific sequences for the ß2-microglobulin and IL6 PCR primers. PCR was performed, and the PCR products were separated by electrophoresis on a 1% agarose gel and visualised after ethidium bromide staining under ultraviolet light. The concentration of st-DNA that gave an amount of PCR product equal to that of the cellular DNA ß2-microglobulin or IL6 PCR products, respectively, was determined. The intensity of the bands was quantified by densitometry. The density of the st-DNA was expressed as a percentage of the total density of st-DNA and cellular cDNA. Results are presented in the absence of A77-1726, and the presence of 10 µM and 100 µM A77-1726.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera P., Haagsma C. J., Boerbooms A. M., Van Riel P. L., Borm G. F., Van de Putte L. B., Van der Meer J. W. Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol. 1995 Aug;34(8):747–755. doi: 10.1093/rheumatology/34.8.747. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N., Santhanam U., Lau L. L., Tatter S. B., Ghrayeb J., Rivelis M., Steinman R. M., Sehgal P. B., May L. T. IL-6/IFN-beta 2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J Immunol. 1989 Oct 1;143(7):2153–2159. [PubMed] [Google Scholar]
- Breedveld F. C., Dayer J. M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000 Nov;59(11):841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Edwin S. L., Cronstein Bruce N. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002 Mar 19;4(4):266–273. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A. S., Finnegan A., Jiang X., Gebel H., Sankary H. N., Foster P., Williams J. W. Leflunomide, a novel immunosuppressive agent. The mechanism of inhibition of T cell proliferation. Transplantation. 1993 Jun;55(6):1361–1366. doi: 10.1097/00007890-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Déage V., Burger D., Dayer J. M. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998 Dec;9(4):663–668. [PubMed] [Google Scholar]
- Emery P., Breedveld F. C., Lemmel E. M., Kaltwasser J. P., Dawes P. T., Gömör B., Van Den Bosch F., Nordström D., Bjorneboe O., Dahl R. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39(6):655–665. doi: 10.1093/rheumatology/39.6.655. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Herrmann M. L., Frangou C. G., Wahl G. M., Morris R. E., Strand V., Kirschbaum B. J. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999 Dec;93(3):198–208. doi: 10.1006/clim.1999.4777. [DOI] [PubMed] [Google Scholar]
- Gerards A. H., de Lathouder S., de Groot E. R., Dijkmans B. A. C., Aarden L. A. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxford) 2003 May 30;42(10):1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- Herrmann M. L., Schleyerbach R., Kirschbaum B. J. Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology. 2000 May;47(2-3):273–289. doi: 10.1016/s0162-3109(00)00191-0. [DOI] [PubMed] [Google Scholar]
- Hoskin D. W., Taylor R. M., Makrigiannis A. P., James H., Lee T. D. Dose-dependent enhancing and inhibitory effects of A77 1726 (leflunomide) on cytotoxic T lymphocyte induction. Int J Immunopharmacol. 1998 Sep;20(9):505–513. doi: 10.1016/s0192-0561(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Hoskin D. W., Taylor R. M., Makrigiannis A. P., James H., Lee T. D. Dose-dependent enhancing and inhibitory effects of A77 1726 (leflunomide) on cytotoxic T lymphocyte induction. Int J Immunopharmacol. 1998 Sep;20(9):505–513. doi: 10.1016/s0192-0561(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Jankovic V., Samardzic T., Stosic-Grujicic S., Popadic D., Trajkovic V. Cell-specific inhibition of inducible nitric oxide synthase activation by leflunomide. Cell Immunol. 2000 Feb 1;199(2):73–80. doi: 10.1006/cimm.1999.1600. [DOI] [PubMed] [Google Scholar]
- Kloppenburg M., Brinkman B. M., de Rooij-Dijk H. H., Miltenburg A. M., Daha M. R., Breedveld F. C., Dijkmans B. A., Verweij C. The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996 Apr;40(4):934–940. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan M. C., Reece R. J., Barg E. C., Smeets T. J., Farnell J., Rosenburg R., Veale D. J., Breedveld F. C., Emery P., Tak P. P. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000 Aug;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kraan M. C., de Koster B. M., Elferink J. G., Post W. J., Breedveld F. C., Tak P. P. Inhibition of neutrophil migration soon after initiation of treatment with leflunomide or methotrexate in patients with rheumatoid arthritis: findings in a prospective, randomized, double-blind clinical trial in fifteen patients. Arthritis Rheum. 2000 Jul;43(7):1488–1495. doi: 10.1002/1529-0131(200007)43:7<1488::AID-ANR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lang R., Wagner H., Heeg K. Differential effects of the immunosuppressive agents cyclosporine and leflunomide in vivo. Leflunomide blocks clonal T cell expansion yet allows production of lymphokines and manifestation of T cell-mediated shock. Transplantation. 1995 Feb 15;59(3):382–389. [PubMed] [Google Scholar]
- Manna S. K., Aggarwal B. B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J Immunol. 1999 Feb 15;162(4):2095–2102. [PubMed] [Google Scholar]
- Manna S. K., Mukhopadhyay A., Aggarwal B. B. Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J Immunol. 2000 Nov 15;165(10):5962–5969. doi: 10.4049/jimmunol.165.10.5962. [DOI] [PubMed] [Google Scholar]
- Montesinos M. Carmen, Desai Avani, Delano Dave, Chen Jiang-Fan, Fink J. Stephen, Jacobson Marlene A., Cronstein Bruce N. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003 Jan;48(1):240–247. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- Morris R. E. New small molecule immunosuppressants for transplantation: review of essential concepts. J Heart Lung Transplant. 1993 Nov-Dec;12(6 Pt 2):S275–S286. [PubMed] [Google Scholar]
- Rozman Blaz. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet. 2002;41(6):421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- Rückemann K., Fairbanks L. D., Carrey E. A., Hawrylowicz C. M., Richards D. F., Kirschbaum B., Simmonds H. A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem. 1998 Aug 21;273(34):21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- Schuerwegh A. J., van Offel J. F., Bridts C. H., Stevens W. J., De Clerck L. S. Influence of longterm therapy with methotrexate and low dose corticosteroids on type 1 and type 2 cytokine production in CD4+ and CD8+ T lymphocytes of patients with rheumatoid arthritis. J Rheumatol. 2001 Aug;28(8):1793–1799. [PubMed] [Google Scholar]
- Siemasko K., Chong A. S., Jäck H. M., Gong H., Williams J. W., Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998 Feb 15;160(4):1581–1588. [PubMed] [Google Scholar]
- Smolen J. S., Emery P. Efficacy and safety of leflunomide in active rheumatoid arthritis. Rheumatology (Oxford) 2000 Jun;39 (Suppl 1):48–56. doi: 10.1093/oxfordjournals.rheumatology.a031495. [DOI] [PubMed] [Google Scholar]
- Strand V., Cohen S., Schiff M., Weaver A., Fleischmann R., Cannon G., Fox R., Moreland L., Olsen N., Furst D. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999 Nov 22;159(21):2542–2550. doi: 10.1001/archinte.159.21.2542. [DOI] [PubMed] [Google Scholar]
- Straub R. H., Müller-Ladner U., Lichtinger T., Schölmerich J., Menninger H., Lang B. Decrease of interleukin 6 during the first 12 months is a prognostic marker for clinical outcome during 36 months treatment with disease-modifying anti-rheumatic drugs. Br J Rheumatol. 1997 Dec;36(12):1298–1303. doi: 10.1093/rheumatology/36.12.1298. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kremer J. M., Coblyn J. S., Maier A. L., Helfgott S. M., Morrell M., Byrne V. M., Kaymakcian M. V., Strand V. Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum. 1999 Jul;42(7):1322–1328. doi: 10.1002/1529-0131(199907)42:7<1322::AID-ANR4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Zielinski T., Müller H. J., Bartlett R. R. Effects of leflunomide (HWA 486) on expression of lymphocyte activation markers. Agents Actions. 1993;38(Spec No):C80–C82. doi: 10.1007/BF01991144. [DOI] [PubMed] [Google Scholar]
- de Lathouder Sacha, Gerards Andreas H., de Groot Els R., Valkhof Marijke, Aarden Lucien A. Mycophenolic acid and methotrexate inhibit lymphocyte cytokine production via different mechanisms. Eur Cytokine Netw. 2002 Jul-Sep;13(3):317–323. [PubMed] [Google Scholar]
- van Ede A. E., Laan R. F. J. M., De Abreu R. A., Stegeman A. B. J., van de Putte L. B. A. Purine enzymes in patients with rheumatoid arthritis treated with methotrexate. Ann Rheum Dis. 2002 Dec;61(12):1060–1064. doi: 10.1136/ard.61.12.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]


