Abstract
Objectives: To assess the possible presence in patients with rheumatoid arthritis (RA) of autoantibodies recognising citrullinated peptides derived from type I and II collagens.
Methods: Firstly, the binding of four pairs of synthetic peptides (arginine-containing and artificially citrullinated forms) related to different regions of human type II collagen were tested with sera from 120 patients with RA and 81 controls. Secondly, two similar pairs of peptides related to the carboxy terminal telopeptides of the α1 and α2 chains of human type I collagen were tested.
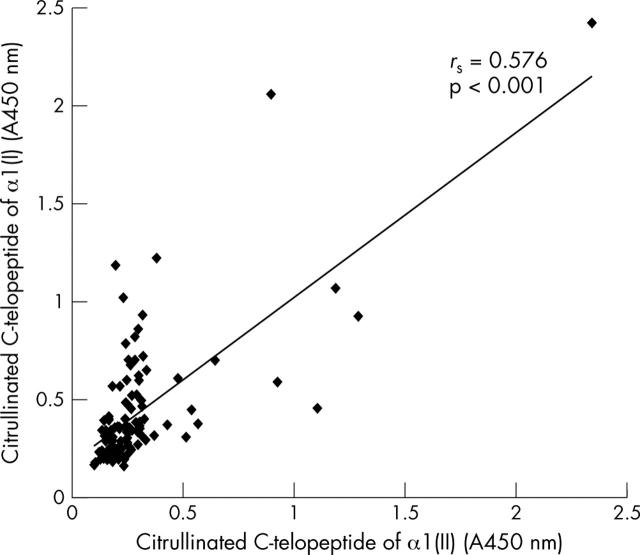
Results: 42–53% of the RA sera showed increased binding of arginine peptides related to type II collagen. However, 12 RA sera bound the citrullinated form of the α1(II) telopeptide more strongly than the corresponding arginine peptide. 20 RA sera bound the citrullinated carboxytelopeptide from the α1 chain of type I collagen (α1(I) telopeptide) more strongly than the respective arginine peptide. The correlation between the autoantibodies to type I and II collagen telopeptides was rs = 0.576, p<0.001. Anti-cyclic citrullinated peptide (anti-CCP) assay was positive in 71/120 (59%) patients with RA. An anti-CCP assay detects a different subgroup of antibodies than anti-telopeptide assays. However, both anti-telopeptide and anti-CCP antibodies were increased in patients with RA.
Conclusion: Some patients with RA were identified whose sera contained antibodies that specifically bound citrullinated peptides related to the carboxy terminal telopeptides of the α1 and α2 chains of type I collagen and the α1 chains of type II collagen (sequences YYXA, FYXA, and YMXA, where X stands for citrulline).
Full Text
The Full Text of this article is available as a PDF (147.0 KB).
Figure 1.
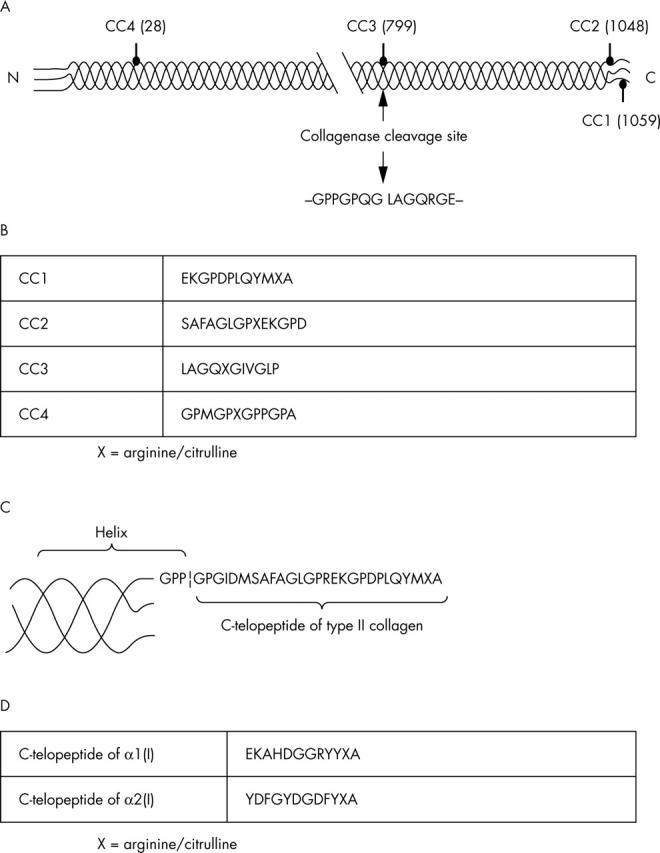
Sequences and localisations of the synthetic peptides used in the study. (A) Localisation of the peptides CC1-CC4 in human type II collagen; the numbers in brackets refer to arginine residues. (B) Sequences of the peptides CC1-CC4. (C) Detailed structure of the carboxy terminal telopeptide of type II collagen; the 12 carboxy terminal amino acids represent the peptide CC1. (D) Sequences of the peptides related to the carboxy terminal telopeptides of the α1 and α2 chains of human type I collagen.
Figure 5.
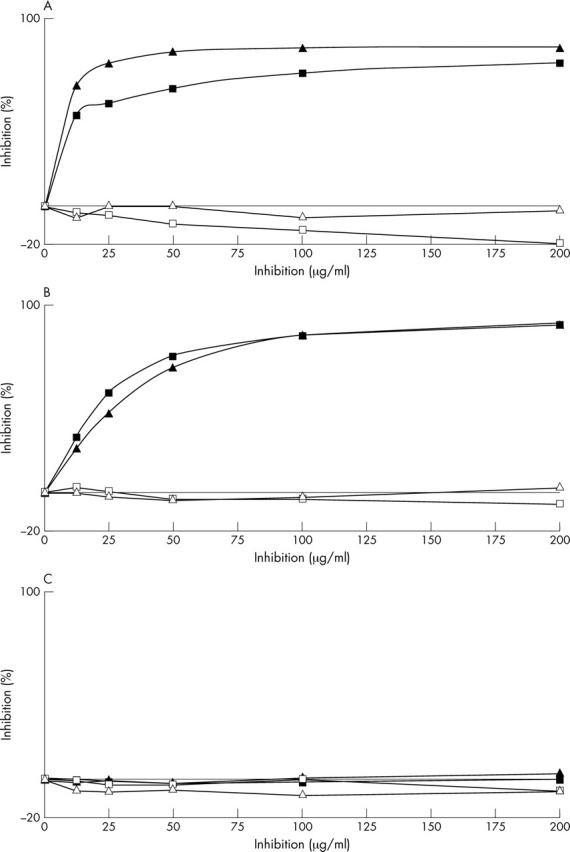
Competition assays. One human serum sample was tested in three different ELISAs: anti-carboxytelopeptides of the α1 chain of type I (A) and II collagens (B) and anti-CCP assays (C). The inhibitors were EKAHDGGRYYRA (open triangles), EKAHDGGRYYXA (closed triangles), EKGPDPLQYMRA (open squares), and EKGPDPLQYMXA (closed squares).
Figure 4.
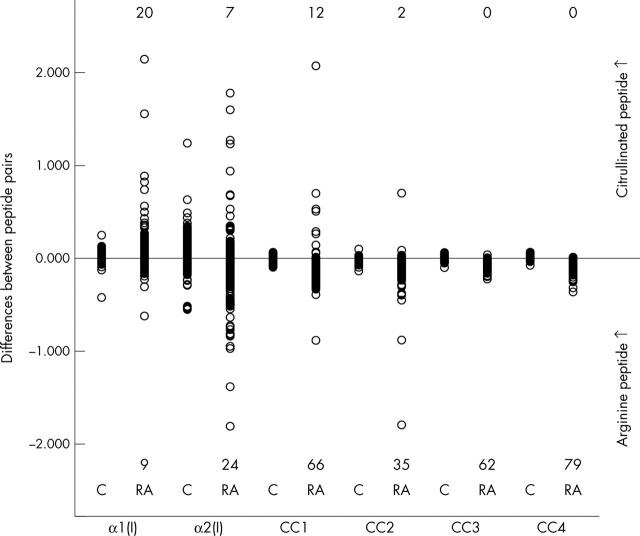
Differences in absorbance between the corresponding citrulline and arginine peptides in the patients with RA and controls for all peptide pairs tested. C = controls, RA = patients with rheumatoid arthritis, each circle represents one serum sample. The circles in the upper part indicate the sera that bind citrullinated peptide more strongly than arginine peptide and vice versa for those in the lower part. The figures in the upper and lower parts indicate the numbers of the cases with preferential binding to either citrullinated or arginine peptide, respectively (exceeding ±2SD of the differences of control sera).
Figure 2.
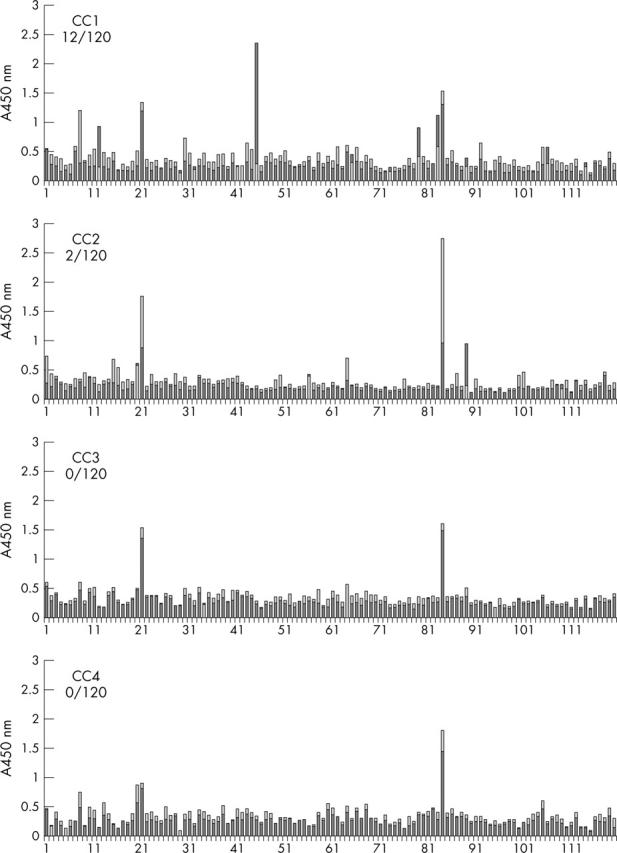
Binding of the peptides CC1 to CC4 to the individual sera of 120 patients with RA. The numbers on the abscissa refer to the serum samples; normal peptides are shown as light grey columns and citrullinated peptides as dark grey columns. The ordinate shows the absorbance measured. For the peptides of CC3 and CC4, no serum bound more strongly to the citrullinated peptide than to the normal peptide. For the CC2 peptide two cases and for the CC1 peptide 12 cases of preferential binding to the citrullinated peptide form were seen.
Figure 3.
Correlation between the bindings (absorbance units) of the carboxy terminal telopeptides of the α1 chains of type I and II collagens to the sera from patients with RA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Clague R. B., Shaw M. J., Holt P. J. Incidence and correlation between serum IgG and IgM antibodies to native type II collagen in patients with chronic inflammatory arthritis. Ann Rheum Dis. 1981 Feb;40(1):6–10. doi: 10.1136/ard.40.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbal-Neuhauser E., Durieux J. J., Arnaud M., Dalbon P., Sebbag M., Vincent C., Simon M., Senshu T., Masson-Bessière C., Jolivet-Reynaud C. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999 Jan 1;162(1):585–594. [PubMed] [Google Scholar]
- Holmdahl Rikard, Bockermann Robert, Bäcklund Johan, Yamada Hisakata. The molecular pathogenesis of collagen-induced arthritis in mice--a model for rheumatoid arthritis. Ageing Res Rev. 2002 Feb;1(1):135–147. doi: 10.1016/s0047-6374(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Masson-Bessière C., Sebbag M., Girbal-Neuhauser E., Nogueira L., Vincent C., Senshu T., Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001 Mar 15;166(6):4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- NIENHUIS R. L., MANDEMA E. A NEW SERUM FACTOR IN PATIENTS WITH RHEUMATOID ARTHRITIS; THE ANTIPERINUCLEAR FACTOR. Ann Rheum Dis. 1964 Jul;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielen Markus M. J., van Schaardenburg Dirkjan, Reesink Henk W., van de Stadt Rob J., van der Horst-Bruinsma Irene E., de Koning Margret H. M. T., Habibuw Moud R., Vandenbroucke Jan P., Dijkmans Ben A. C. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004 Feb;50(2):380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- Nijenhuis Suzanne, Zendman Albert J. W., Vossenaar Erik R., Pruijn Ger J. M., vanVenrooij Walther J. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta. 2004 Dec;350(1-2):17–34. doi: 10.1016/j.cccn.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Nomura K. Specificity and mode of action of the muscle-type protein-arginine deiminase. Arch Biochem Biophys. 1992 Mar;293(2):362–369. doi: 10.1016/0003-9861(92)90407-n. [DOI] [PubMed] [Google Scholar]
- Orgel J. P., Wess T. J., Miller A. The in situ conformation and axial location of the intermolecular cross-linked non-helical telopeptides of type I collagen. Structure. 2000 Feb 15;8(2):137–142. doi: 10.1016/s0969-2126(00)00089-7. [DOI] [PubMed] [Google Scholar]
- Rantapä-Dahlqvist Solbritt, de Jong Ben A. W., Berglin Ewa, Hallmans Göran, Wadell Göran, Stenlund Hans, Sundin Ulf, van Venrooij Walther J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003 Oct;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- Rowley M., Tait B., Mackay I. R., Cunningham T., Phillips B. Collagen antibodies in rheumatoid arthritis. Significance of antibodies to denatured collagen and their association with HLA-DR4. Arthritis Rheum. 1986 Feb;29(2):174–184. doi: 10.1002/art.1780290204. [DOI] [PubMed] [Google Scholar]
- Schellekens G. A., Visser H., de Jong B. A., van den Hoogen F. H., Hazes J. M., Breedveld F. C., van Venrooij W. J. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000 Jan;43(1):155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schellekens G. A., de Jong B. A., van den Hoogen F. H., van de Putte L. B., van Venrooij W. J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998 Jan 1;101(1):273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Huffstutter E. H., Townes A. S., Kang A. H. Incidence and specificity of antibodies to types I, II, III, IV, and V collagen in rheumatoid arthritis and other rheumatic diseases as measured by 125I-radioimmunoassay. Arthritis Rheum. 1983 Jul;26(7):832–840. doi: 10.1002/art.1780260703. [DOI] [PubMed] [Google Scholar]
- Visser Henk, le Cessie Saskia, Vos Koen, Breedveld Ferdinand C., Hazes Johanna M. W. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002 Feb;46(2):357–365. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- Vossenaar Erik R., Després Normand, Lapointe Elvy, van der Heijden Annemarie, Lora Maximillian, Senshu Tatsuo, van Venrooij Walther J., Ménard Henri A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004 Feb 5;6(2):R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Ryo, Suzuki Akari, Chang Xiaotian, Yamamoto Kazuhiko. Citrullinated proteins in rheumatoid arthritis. Front Biosci. 2005 Jan 1;10:54–64. doi: 10.2741/1506. [DOI] [PubMed] [Google Scholar]
- Young B. J., Mallya R. K., Leslie R. D., Clark C. J., Hamblin T. J. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979 Jul 14;2(6182):97–99. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boekel Martinus A. M., Vossenaar Erik R., van den Hoogen Frank H. J., van Venrooij Walther J. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2001 Nov 6;4(2):87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen F. A., Linn-Rasker S. P., van Venrooij W. J., de Jong B. A., Breedveld F. C., Verweij C. L., Toes R. E. M., Huizinga T. W. J. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004 Mar;50(3):709–715. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Pruijn G. J. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000 May 24;2(4):249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]