Abstract
Objective: To investigate the intracellular and soluble cytokine levels and T cell subsets in peripheral blood of patients with active and inactive polymyositis and dermatomyositis.
Methods: The frequencies of T and B lymphocytes, T helper (Th), and T cytotoxic (Tc) cells and of interferon γ (IFNγ), interleukin (IL)4, and IL10 expression of CD4+ or CD8+ cells were determined by flow cytometry. The concentrations of soluble cytokines were measured with commercial enzyme linked immunosorbent assays.
Results: In active dermatomyositis there was a decreased percentage of T (CD3+) lymphocytes and Tc (CD8+) lymphocytes, decreased IFNγ expression of CD4+ and CD8+ cells, but an increase in B and IL4 producing CD4+ lymphocyte frequencies. These prominent changes disappeared in the inactive stage of the disease. In polymyositis no significant change in these lymphocyte subsets or in intracellular cytokine expression could be detected in either the active or the inactive form. The frequency of IL4+/IFNγ+ Th cells was calculated and a significantly increased Th2/Th1 frequency was found in active dermatomyositis, and a decreased frequency in inactive dermatomyositis, compared with the control population.
Conclusions: There appears to be a difference between polymyositis and dermatomyositis in the level of peripheral blood lymphocytes and their intracellular cytokine content. These findings provide further evidence for a difference in the pathogenesis of polymyositis and dermatomyositis.
Full Text
The Full Text of this article is available as a PDF (84.9 KB).
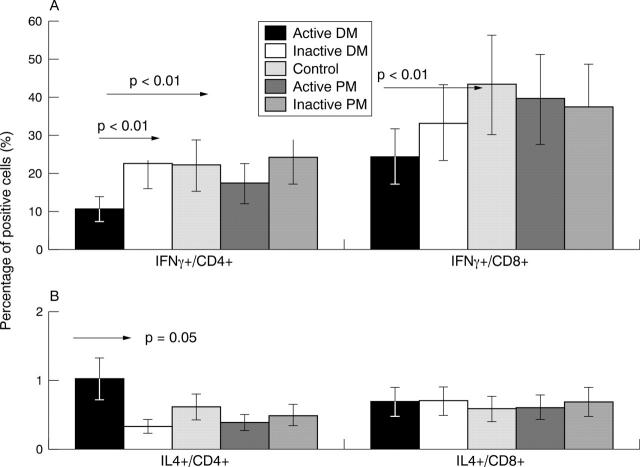
Figure 1.
Expression of intracellular interferon γ (IFNγ) (panel A) and interleukin 4 (IL4) (panel B) in stimulated peripheral T helper (Th) and T cytotoxic (Tc) cells of patients with dermatomyositis (DM) and polymyositis (PM). The samples were measured by flow cytometry, and lymphocytes were identified on their scatter properties and CD4/CD8 positivities.
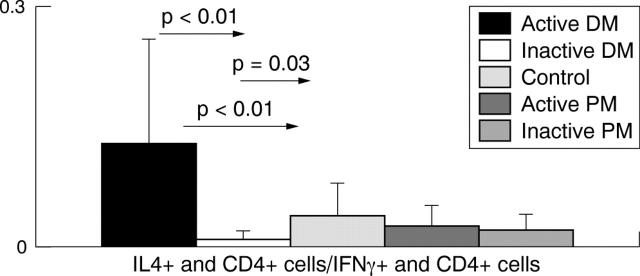
Figure 2.
Calculated Th2/Th1 frequency (Th2: IL4+/CD4+; Th1: IFNγ+/CD4+) in active and inactive polymyositis (PM) and dermatomyositis (DM). IFN, interferon; IL, interleukin; Th, T helper cell.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Engel A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. IV: Cell-mediated cytotoxicity and muscle fiber necrosis. Ann Neurol. 1988 Feb;23(2):168–173. doi: 10.1002/ana.410230210. [DOI] [PubMed] [Google Scholar]
- Bender A., Ernst N., Iglesias A., Dornmair K., Wekerle H., Hohlfeld R. T cell receptor repertoire in polymyositis: clonal expansion of autoaggressive CD8+ T cells. J Exp Med. 1995 May 1;181(5):1863–1868. doi: 10.1084/jem.181.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste O., Chérin P., Maisonobe T., Merat R., Chosidow O., Mouthon L., Guillevin L., Flahault A., Burland M. C., Klatzmann D. Severe perturbations of the blood T cell repertoire in polymyositis, but not dermatomyositis patients. J Immunol. 2001 Sep 15;167(6):3521–3529. doi: 10.4049/jimmunol.167.6.3521. [DOI] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Brouwer R., Hengstman G. J., Vree Egberts W., Ehrfeld H., Bozic B., Ghirardello A., Grøndal G., Hietarinta M., Isenberg D., Kalden J. R. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis. 2001 Feb;60(2):116–123. doi: 10.1136/ard.60.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein D. M., O'Gorman M. R., Pachman L. M. Correlations between change in disease activity and changes in peripheral blood lymphocyte subsets in patients with juvenile dermatomyositis. J Rheumatol. 1997 Sep;24(9):1830–1832. [PubMed] [Google Scholar]
- Engel A. G., Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986 Jul;17(7):704–721. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- Gabay C., Gay-Croisier F., Roux-Lombard P., Meyer O., Maineti C., Guerne P. A., Vischer T., Dayer J. M. Elevated serum levels of interleukin-1 receptor antagonist in polymyositis/dermatomyositis. A biologic marker of disease activity with a possible role in the lack of acute-phase protein response. Arthritis Rheum. 1994 Dec;37(12):1744–1751. doi: 10.1002/art.1780371206. [DOI] [PubMed] [Google Scholar]
- Guo J., Rapoport B., McLachlan S. M. Balance of Th1/Th2 cytokines in thyroid autoantibody synthesis in vitro. Autoimmunity. 1999;30(1):1–9. doi: 10.3109/08916939908994754. [DOI] [PubMed] [Google Scholar]
- Hagiwara E., Adams E. M., Plotz P. H., Klinman D. M. Abnormal numbers of cytokine producing cells in patients with polymyositis and dermatomyositis. Clin Exp Rheumatol. 1996 Sep-Oct;14(5):485–491. [PubMed] [Google Scholar]
- Hagiwara E., Gourley M. F., Lee S., Klinman D. K. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996 Mar;39(3):379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Goebels N., Engel A. G. Cellular mechanisms in inflammatory myopathies. Baillieres Clin Neurol. 1993 Nov;2(3):617–635. [PubMed] [Google Scholar]
- Iannone F., Cauli A., Yanni G., Kingsley G. H., Isenberg D. A., Corrigall V., Panayi G. S. T-lymphocyte immunophenotyping in polymyositis and dermatomyositis. Br J Rheumatol. 1996 Sep;35(9):839–845. doi: 10.1093/rheumatology/35.9.839. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Koarada S., Tada Y., Ushiyama O., Morito F., Suzuki N., Ohta A., Horiuchi T., Miyake K., Nagasawa K. Difference in B cell activation between dermatomyositis and polymyositis: analysis of the expression of RP105 on peripheral blood B cells. Ann Rheum Dis. 2001 Dec;60(12):1137–1140. doi: 10.1136/ard.60.12.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg I., Ulfgren A. K., Nyberg P., Andersson U., Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997 May;40(5):865–874. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Love L. A., Barbieri S. A., Balow J. E., Plotz P. H. Lymphocyte activation markers in idiopathic myositis: changes with disease activity and differences among clinical and autoantibody subgroups. Clin Exp Immunol. 1990 Sep;81(3):373–379. doi: 10.1111/j.1365-2249.1990.tb05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P., Wikman A. L., Nennesmo I., Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol. 2000 Apr;27(4):940–948. [PubMed] [Google Scholar]
- O'Gorman M. R., Corrochano V., Roleck J., Donovan M., Pachman L. M. Flow cytometric analyses of the lymphocyte subsets in peripheral blood of children with untreated active juvenile dermatomyositis. Clin Diagn Lab Immunol. 1995 Mar;2(2):205–208. doi: 10.1128/cdli.2.2.205-208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon T. P., Dalakas M. C., Plotz P. H., Miller F. W. Predominant TCR-alpha beta variable and joining gene expression by muscle-infiltrating lymphocytes in the idiopathic inflammatory myopathies. J Immunol. 1994 Mar 1;152(5):2569–2576. [PubMed] [Google Scholar]
- Oliver J. C., Bland L. A., Oettinger C. W., Arduino M. J., McAllister S. K., Aguero S. M., Favero M. S. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993 Apr;12(2):115–120. [PubMed] [Google Scholar]
- Shirai A., Holmes K., Klinman D. Detection and quantitation of cells secreting IL-6 under physiologic conditions in BALB/c mice. J Immunol. 1993 Feb 1;150(3):793–799. [PubMed] [Google Scholar]
- Sugiura T., Kawaguchi Y., Harigai M., Takagi K., Ohta S., Fukasawa C., Hara M., Kamatani N. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J Immunol. 2000 Jun 15;164(12):6593–6600. doi: 10.4049/jimmunol.164.12.6593. [DOI] [PubMed] [Google Scholar]
- Targoff I. N. Humoral immunity in polymyositis/dermatomyositis. J Invest Dermatol. 1993 Jan;100(1):116S–123S. doi: 10.1111/1523-1747.ep12356607. [DOI] [PubMed] [Google Scholar]
- Targoff I. N. Immunologic aspects of myositis. Curr Opin Rheumatol. 1989 Dec;1(4):432–442. doi: 10.1097/00002281-198901040-00004. [DOI] [PubMed] [Google Scholar]
- Urbano-Márquez A., Casademont J., Grau J. M. Polymyositis/dermatomyositis: the current position. Ann Rheum Dis. 1991 Mar;50(3):191–195. doi: 10.1136/ard.50.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]