Abstract
Objective: To determine clinical and immunological correlates of high dose chemotherapy (HDC) + autologous stem cell transplantation (ASCT) in patients with severe rheumatoid arthritis (RA), refractory to conventional treatment.
Methods: Serial samples of peripheral blood and synovial tissue were obtained from seven patients with RA treated with HDC and autologous peripheral blood grafts enriched for CD34+ cells. Disease activity was assessed with the Disease Activity Score (DAS), serum concentrations of C reactive protein (CRP), and human immunoglobulin (HIg) scans, and the extent of immunoablation was determined by immunophenotyping of peripheral blood mononuclear cells, and immunohistochemistry and double immunofluorescence of synovium.
Results: Clinical responders (n = 5) had a larger number of cells at baseline expressing CD3, CD4, CD27, CD45RA, CD45RB, and CD45RO in synovium (p<0.05), higher activity on HIg scans (p = 0.08), and a trend towards higher concentrations of CRP in serum than non-responders (n = 2). Subsequent remissions and relapses in responders paralleled reduction and re-expression, respectively, of T cell markers. A relatively increased expression of CD45RB and CD45RO on synovial CD3+ T cells was seen after HDC + ASCT. No correlations were found between DAS and changes in B cells or macrophage infiltration or synoviocytes.
Conclusions: HDC + ASCT results in profound but incomplete immunoablation of both the memory and naïve T cell compartment, which is associated with longlasting clinical responses in most patients. The findings provide strong circumstantial evidence for a role of T cells in established RA, and demonstrate a role for the synovium in post-transplantation T cell reconstitution.
Full Text
The Full Text of this article is available as a PDF (281.2 KB).
Figure 1.
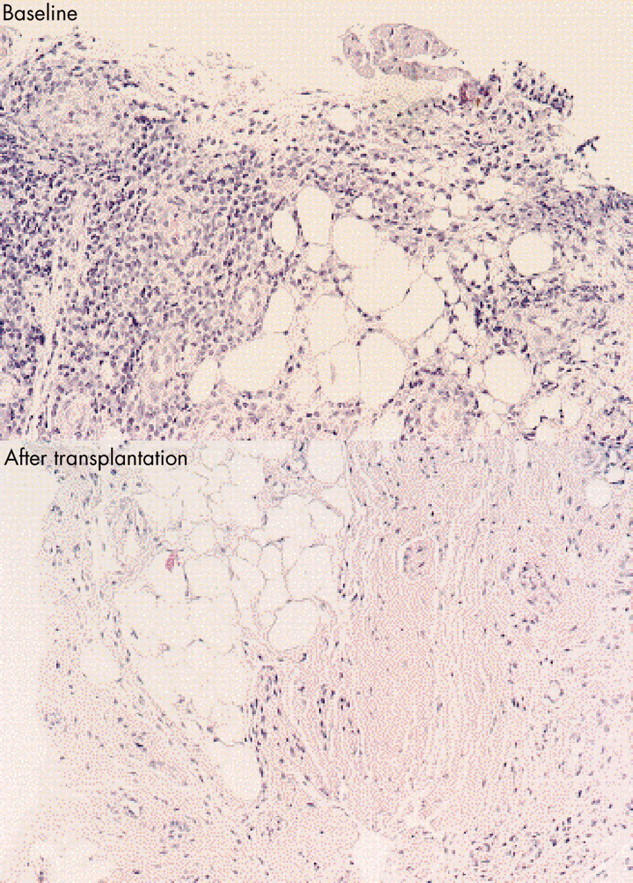
Synovial tissue taken from patient No 2 (table 1) before and 3 months after HDC + ASCT. Infiltration with numerous lymphocytes and plasma cells before stem cell transplantation, which were almost absent after the transplantation.
Figure 2.
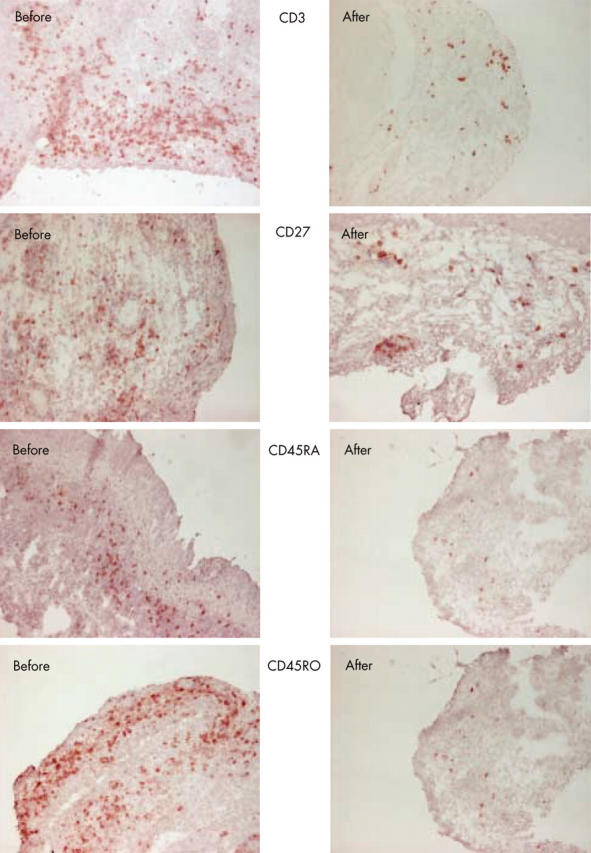
Immunohistochemically stained synovial tissue in a responder with CD3, CD27, CD45RA, and CD45RO, before and 3 months after HDC + ASCT.
Figure 3.
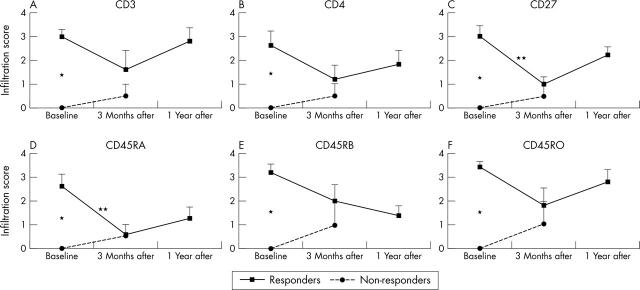
Semiquantitative infiltration scores before, 3 months after, and 1 year after HDC + ASCT of responders and non-responders for CD3, CD4, CD27, CD45RA, CD45RB, and CD45RO. *p<0.05, significant baseline difference between responders and non-responders; **p<0.05, significant decrease at 3 months after transplantation in responders CD45RA and CD27.
Figure 4.
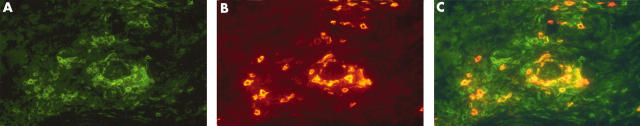
Co-expression of CD3 and CD45RO in RA synovial tissue before transplantation in a responder. CD3 (FITC = green) and CD45RO (TRITC = red) were detected using immunofluorescence techniques. (A) Rheumatoid synovial tissue showing CD3+ cell infiltrate. (B) Rheumatoid synovial tissue showing CD45RO+ cell infiltrate. (C) Double positive cells as depicted by yellow and orange, showing numerous double positive and only a few CD45RO single positive cells (red).
Figure 6.
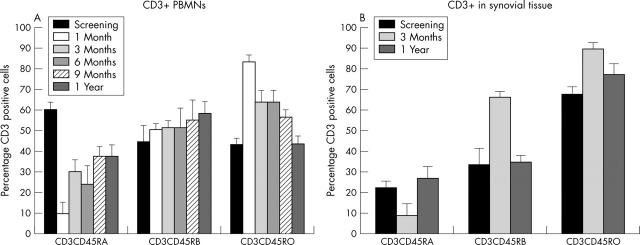
The mean number of CD45RA, CD45RB, and CD45RO expressed as a percentage of CD3+cells. (A) Immunofluorescence double staining of peripheral blood mononuclear cells with CD3 plus CD45RA, CD45RB, or CD45RO in the five responders at screening and at 1, 3, 6, 9, and 12 months after HDC + ASCT. Results expressed as the percentage of CD3 cells expressing the CD45R isoform. (B) Immunofluorescence double staining of synovial cells with CD3 plus CD45RA, CD45RB, or CD45RO in the five responders at screening and 3 and 12 months after HDC + ASCT. Results expressed as the percentage of CD3 cells expressing the CD45R isoform.
Figure 5.
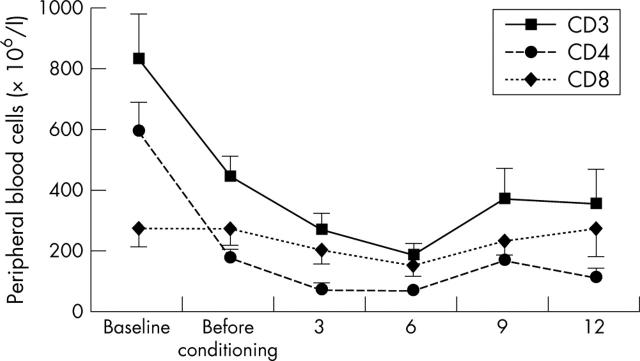
Absolute cell count in peripheral blood mononuclear cells in the five responders for CD3, CD4, and CD8. Absolute cell numbers were calculated by multiplying absolute lymphocyte count (106/l (SEM)) by the percentage of each subset determined by flow cytometry after isolation of peripheral blood mononuclear cells by density gradient centrifugation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bingham Sarah, Veale Douglas, Fearon Ursula, Isaacs John D., Morgan Gareth, Emery Paul, McGonagle Dennis, Reece Richard, Clague Roy, Snowden John A. High-dose cyclophosphamide with stem cell rescue for severe rheumatoid arthritis: short-term efficacy correlates with reduction of macroscopic and histologic synovitis. Arthritis Rheum. 2002 Mar;46(3):837–839. doi: 10.1002/art.10093. [DOI] [PubMed] [Google Scholar]
- Brinkman Danielle M. C., ten Cate Rebecca, Vossen Jaak M., Smeets Tom J. M., Kraan Maarten C., Tak Paul P. Decrease in synovial cellularity and cytokine expression after autologous stem cell transplantation in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002 Apr;46(4):1121–1123. doi: 10.1002/art.10186. [DOI] [PubMed] [Google Scholar]
- Burt R. K., Georganas C., Schroeder J., Traynor A., Stefka J., Schuening F., Graziano F., Mineishi S., Brush M., Fishman M. Autologous hematopoietic stem cell transplantation in refractory rheumatoid arthritis: sustained response in two of four patients. Arthritis Rheum. 1999 Nov;42(11):2281–2285. doi: 10.1002/1529-0131(199911)42:11<2281::AID-ANR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- De Keyser F., Elewaut D., Vermeersch J., De Wever N., Cuvelier C., Veys E. M. The role of T cells in rheumatoid arthritis. Clin Rheumatol. 1995 Sep;14 (Suppl 2):5–9. doi: 10.1007/BF02215850. [DOI] [PubMed] [Google Scholar]
- Dolhain R. J., Ter Haar N. T., De Kuiper R., Nieuwenhuis I. G., Zwinderman A. H., Breedveld F. C., Miltenburg A. M. Distribution of T cells and signs of T-cell activation in the rheumatoid joint: implications for semiquantitative comparative histology. Br J Rheumatol. 1998 Mar;37(3):324–330. doi: 10.1093/rheumatology/37.3.324. [DOI] [PubMed] [Google Scholar]
- Durez P., Toungouz M., Schandené L., Lambermont M., Goldman M. Remission and immune reconstitution after T-cell-depleted stem-cell transplantation for rheumatoid arthritis. Lancet. 1998 Sep 12;352(9131):881–881. doi: 10.1016/S0140-6736(05)60008-6. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Strober S., Solovera J. J., Gandour D., Lane N., Schurman D., Hoppe R. T., Chin R. C., Eugui E. M., Vaughan J. H. Dissection of the mechanisms of immune injury in rheumatoid arthritis, using total lymphoid irradiation. Arthritis Rheum. 1988 Jan;31(1):21–30. doi: 10.1002/art.1780310104. [DOI] [PubMed] [Google Scholar]
- Isaacs J. D., Greer S., Sharma S., Symmons D., Smith M., Johnston J., Waldmann H., Hale G., Hazleman B. L. Morbidity and mortality in rheumatoid arthritis patients with prolonged and profound therapy-induced lymphopenia. Arthritis Rheum. 2001 Sep;44(9):1998–2008. doi: 10.1002/1529-0131(200109)44:9<1998::AID-ART348>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jendro M. C., Ganten T., Matteson E. L., Weyand C. M., Goronzy J. J. Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum. 1995 Sep;38(9):1242–1251. doi: 10.1002/art.1780380912. [DOI] [PubMed] [Google Scholar]
- Joske D. J., Ma D. T., Langlands D. R., Owen E. T. Autologous bone-marrow transplantation for rheumatoid arthritis. Lancet. 1997 Aug 2;350(9074):337–338. doi: 10.1016/s0140-6736(05)63388-0. [DOI] [PubMed] [Google Scholar]
- Koetz K., Bryl E., Spickschen K., O'Fallon W. M., Goronzy J. J., Weyand C. M. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Kansas G. S., Engleman E. G., Hoppe R. T., Kaplan H. S., Strober S. Changes in T-cell subsets in patients with rheumatoid arthritis treated with total lymphoid irradiation. Clin Immunol Immunopathol. 1983 May;27(2):250–260. doi: 10.1016/0090-1229(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Kraan Maarten C., van Kuijk Arno W. R., Dinant Huibert J., Goedkoop Amber Y., Smeets Tom J. M., de Rie Menno A., Dijkmans Ben A. C., Vaishnaw Akshay K., Bos Jan D., Tak Paul P. Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum. 2002 Oct;46(10):2776–2784. doi: 10.1002/art.10543. [DOI] [PubMed] [Google Scholar]
- Lowenthal R. M., Cohen M. L., Atkinson K., Biggs J. C. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol. 1993 Jan;20(1):137–140. [PubMed] [Google Scholar]
- Nanki T., Hayashida K., El-Gabalawy H. S., Suson S., Shi K., Girschick H. J., Yavuz S., Lipsky P. E. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000 Dec 1;165(11):6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- Pavletic S. Z., Odell J. R., Pirruccello S. J., Ursick M. M., Haire C. E., Sharp J. G., Kessinger A., Klassen L. W. Intensive immunoablation and autologous blood stem cell transplantation in patients with refractory rheumatoid arthritis: the University of Nebraska experience. J Rheumatol Suppl. 2001 Oct;64:13–20. [PubMed] [Google Scholar]
- Ponchel Frederique, Morgan Ann W., Bingham Sarah J., Quinn Mark, Buch Maya, Verburg Robert J., Henwood Judy, Douglas Susan H., Masurel Aurelie, Conaghan Philip. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002 Aug 8;100(13):4550–4556. doi: 10.1182/blood-2002-03-0671. [DOI] [PubMed] [Google Scholar]
- Salmon M., Gaston J. S. The role of T-lymphocytes in rheumatoid arthritis. Br Med Bull. 1995 Apr;51(2):332–345. doi: 10.1093/oxfordjournals.bmb.a072964. [DOI] [PubMed] [Google Scholar]
- Snowden J. A., Biggs J. C., Milliken S. T., Fuller A., Brooks P. M. A phase I/II dose escalation study of intensified cyclophosphamide and autologous blood stem cell rescue in severe, active rheumatoid arthritis. Arthritis Rheum. 1999 Nov;42(11):2286–2292. doi: 10.1002/1529-0131(199911)42:11<2286::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Snowden John A., Passweg Jakob, Moore John J., Milliken Sam, Cannell Paul, Van Laar Jacob, Verburg Robert, Szer Jeffrey, Taylor Kerry, Joske David. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004 Mar;31(3):482–488. [PubMed] [Google Scholar]
- Tak P. P., van der Lubbe P. A., Cauli A., Daha M. R., Smeets T. J., Kluin P. M., Meinders A. E., Yanni G., Panayi G. S., Breedveld F. C. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995 Oct;38(10):1457–1465. doi: 10.1002/art.1780381012. [DOI] [PubMed] [Google Scholar]
- Verburg R. J., Kruize A. A., van den Hoogen F. H., Fibbe W. E., Petersen E. J., Preijers F., Sont J. K., Barge R. M., Bijlsma J. W., van de Putte L. B. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in patients with rheumatoid arthritis: results of an open study to assess feasibility, safety, and efficacy. Arthritis Rheum. 2001 Apr;44(4):754–760. doi: 10.1002/1529-0131(200104)44:4<754::AID-ANR131>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Verburg R. obert J., Toes René E. M., Fibbe Willem E., Breedveld Ferdinand C., van Laar Jacob M. High dose chemotherapy and autologous hematopoietic stem cell transplantation for rheumatoid arthritis: a review. Hum Immunol. 2002 Aug;63(8):627–637. doi: 10.1016/s0198-8859(02)00414-7. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Gupta R., Morita Y., He X., Pai R., Endres J., Freiberg A., Chung K., Fox D. A. Effector function of resting T cells: activation of synovial fibroblasts. J Immunol. 2001 Feb 15;166(4):2270–2275. doi: 10.4049/jimmunol.166.4.2270. [DOI] [PubMed] [Google Scholar]
- de Bois M. H., Arndt J. W., van der Velde E. A., van der Lubbe P. A., Westedt M. L., Pauwels E. K., Breedveld F. C. 99mTc human immunoglobulin scintigraphy--a reliable method to detect joint activity in rheumatoid arthritis. J Rheumatol. 1992 Sep;19(9):1371–1376. [PubMed] [Google Scholar]
- van Bekkum D. W. Conditioning regimens for the treatment of experimental arthritis with autologous bone marrow transplantation. Bone Marrow Transplant. 2000 Feb;25(4):357–364. doi: 10.1038/sj.bmt.1702153. [DOI] [PubMed] [Google Scholar]
- van Gestel A. M., Prevoo M. L., van 't Hof M. A., van Rijswijk M. H., van de Putte L. B., van Riel P. L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996 Jan;39(1):34–40. doi: 10.1002/art.1780390105. [DOI] [PubMed] [Google Scholar]
- van der Heijde D. M., van 't Hof M., van Riel P. L., van de Putte L. B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993 Mar;20(3):579–581. [PubMed] [Google Scholar]
- van der Pouw Kraan Tineke C. T. M., van Gaalen Floris A., Kasperkovitz Pia V., Verbeet Nicolette L., Smeets Tom J. M., Kraan Maarten C., Fero Mike, Tak Paul-Peter, Huizinga Tom W. J., Pieterman Elsbet. Rheumatoid arthritis is a heterogeneous disease: evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. 2003 Aug;48(8):2132–2145. doi: 10.1002/art.11096. [DOI] [PubMed] [Google Scholar]