Abstract
Background: Collagen induced arthritis (CIA) is an animal model of rheumatoid arthritis (RA) amenable to immunotherapy directed against tumour necrosis factor α (TNFα).
Objective: To evaluate whether local TNF receptor (TNF-R) gene therapy in DBA/1 mice exerts an influence beyond anti-inflammatory effects. Two measures of CIA pathogenesis were investigated—namely, immunity to collagen II (CII) 245–270 peptide (the major immunodominant epitope within bovine CII) and the preferential activation of T cell Vß8.2 variable region receptors in arthritic DBA/1 mice.
Methods: DBA/1 mice received single periarticular injections of media or retroviral vectors containing LacZ or human TNF-R into affected arthritic paws at disease onset. Disease severity was monitored, immune responses towards the immunodominant bovine CII 245–270 and subdominant CII 334–360 peptide epitopes were assessed by ELISA, and T cell Vß usage was analysed by real time polymerase chain reaction for the LacZ transduced, TNF-R, and viral-free media treated control animals. The therapeutic influence of TNF-R gene transduction was compared with other groups at different times after treatment.
Results: Reduced disease severity was seen 15–35 days after treatment, with a concomitant increase in immunity towards the subdominant CII 334–360 peptide epitope rather than the immunodominant CII 245–270 peptide in TNF-R treated animals. Early in the disease, TNF-R treated animals demonstrated a reduction of bias towards the otherwise predominant Vß8.2 T cell subset.
Conclusions: TNF-R gene therapy influences cellular immunity in CIA, leading to overall disease amelioration, thus suggesting that TNF inhibition may have therapeutic potential beyond the control of inflammation in RA.
Full Text
The Full Text of this article is available as a PDF (111.8 KB).
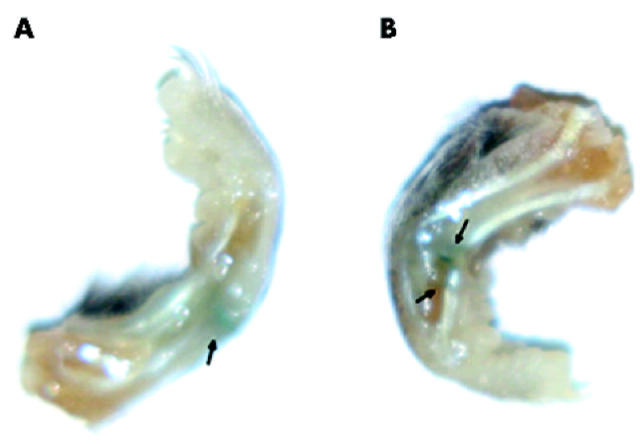
Figure 1.
X-gal stain to show the expression of LacZ in the mouse paws given MFG-LacZ. (A) A paw at 3 days; and (B) 21 days after viral infection.
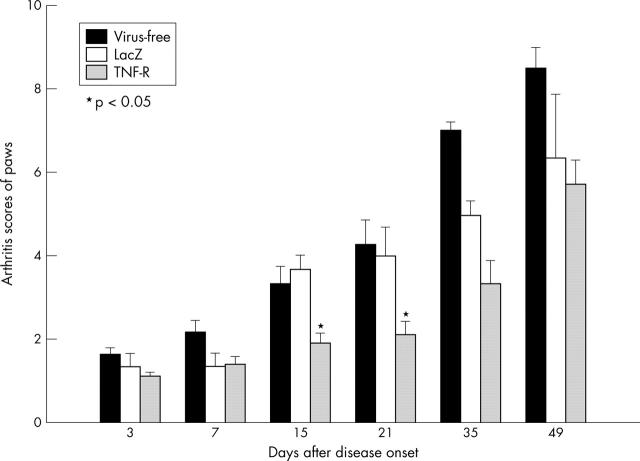
Figure 2.
Influence of a single periarticular administration of retrovirus mediated MOIN-sTNF-Rc-Ig on the clinical progression of CIA: disease activity represented by the mean paw score was visually scored up to 49 days after disease onset. Mean paw score is the overall average of the paw scores of all animals in each group. Data are expressed as mean (SEM) for each group for each time point. Significant differences among the groups are indicated (*p<0. 05). The figure shows normalised mean data from three different individual experiments with similar results.
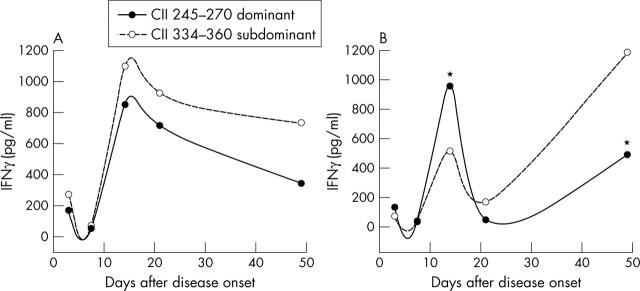
Figure 3.
IFNγ responses to CII 245–270 and CII 330–360 peptides after MOIN-sTNF-Rc-Ig gene therapy: mononuclear cells from lymph nodes of (A) MOIN-sTNF-Rc-Ig or (B) media (control) treated mice were cultured with either 100 µg/µl of immunodominant CII 245–270 peptide or with subdominant CII 330–360 peptide for 72 hours. Cells cultured with media and concanavalin A were used as negative and positive controls respectively (not shown). IFNγ levels in resulting culture supernatants were measured by ELISA to evaluate CII peptide epitope usage. TNF-R treated animals showed an enhanced IFNγ response towards the subdominant epitopes rather than the immunodominant epitopes (A), while the normal response to the dominant epitope was observed in the controls (B). Data are expressed as mean for each group at each time point. n = 5 for each time point for both control and TNF-R treated groups. *p<0.05.
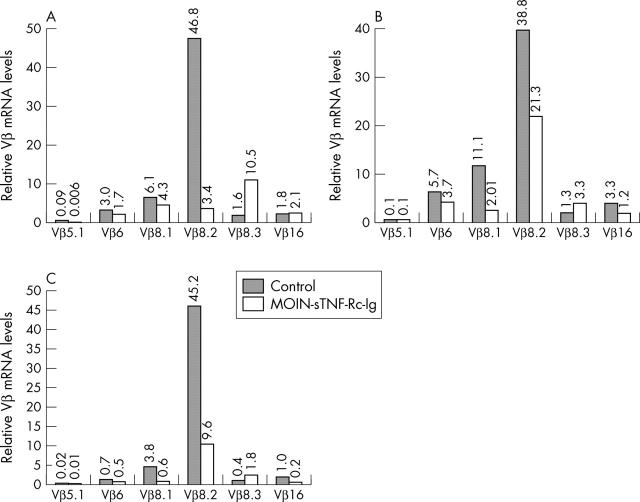
Figure 4.
Comparison of Vß expression levels in joints of arthritic animals: cDNA made from RNA preparations from (A) injected (B) ipsilateral (C) contralateral joints of media or MOIN-sTNF-Rc-Ig injected joints were amplified with different Vß primer pairs using real time PCR. The figure shows the relative mRNA expression levels of the six Vß genes quantitatively analysed normalised against the housekeeping gene 18S rRNA used as an internal control. n = 5 for both control and TNF-R treated groups.
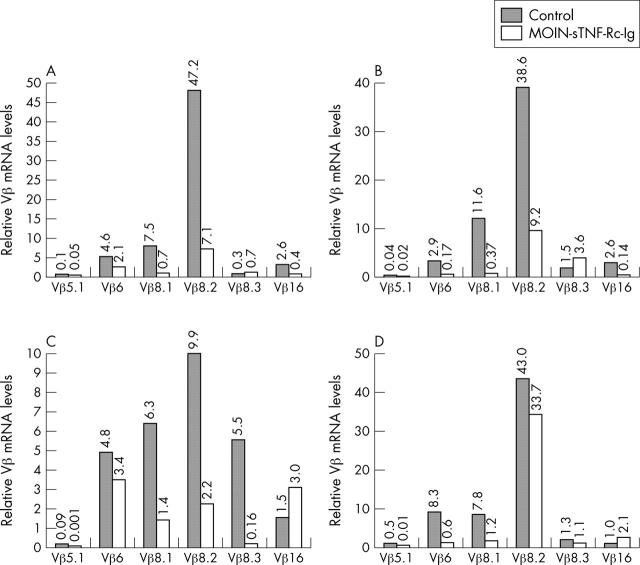
Figure 5.
Local TNF-R treatment affects the TCR Vß repertoire in CIA over time: Vß mRNA levels in the joints of control and MOIN-sTNF-Rc-Ig treated groups were compared by real time RT-PCR at (A) 3; (B) 7; (C) 21; and (D) 49 days after disease onset. cDNA was obtained from RNA preparations from joints of the two groups of mice. Relative Vß gene expression in both groups, normalised against the housekeeping gene 18S rRNA used as an internal control, is represented. n = 5 for both control and TNF-R treated groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995 Feb;38(2):151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Brand D. D., Myers L. K., Terato K., Whittington K. B., Stuart J. M., Kang A. H., Rosloniec E. F. Characterization of the T cell determinants in the induction of autoimmune arthritis by bovine alpha 1(II)-CB11 in H-2q mice. J Immunol. 1994 Mar 15;152(6):3088–3097. [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Butler D. M., Maini R. N., Feldmann M., Brennan F. M. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995 Jul-Dec;6(4):225–230. [PubMed] [Google Scholar]
- Chiocchia G., Boissier M. C., Fournier C. Therapy against murine collagen-induced arthritis with T cell receptor V beta-specific antibodies. Eur J Immunol. 1991 Dec;21(12):2899–2905. doi: 10.1002/eji.1830211202. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Panayi G. S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001 Mar 22;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Londei M. Induction of Th2 cytokines and control of collagen-induced arthritis by nondepleting anti-CD4 Abs. J Immunol. 1996 Sep 15;157(6):2685–2689. [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Bijl H., Woody J. N. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994 Oct 22;344(8930):1125–1127. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Ghivizzani S. C., Lechman E. R., Kang R., Tio C., Kolls J., Evans C. H., Robbins P. D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghivizzani S. C., Lechman E. R., Tio C., Mulé K. M., Chada S., McCormack J. E., Evans C. H., Robbins P. D. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997 Sep;4(9):977–982. doi: 10.1038/sj.gt.3300486. [DOI] [PubMed] [Google Scholar]
- Haqqi T. M., Anderson G. D., Banerjee S., David C. S. Restricted heterogeneity in T-cell antigen receptor V beta gene usage in the lymph nodes and arthritic joints of mice. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1253–1255. doi: 10.1073/pnas.89.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqi T. M., Banerjee S., Anderson G. D., David C. S. RIII S/J (H-2r). An inbred mouse strain with a massive deletion of T cell receptor V beta genes. J Exp Med. 1989 Jun 1;169(6):1903–1909. doi: 10.1084/jem.169.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqqi T. M., Banerjee S., Jones W. L., Anderson G., Behlke M. A., Loh D. Y., Luthra H. S., David C. S. Identification of T-cell receptor V beta deletion mutant mouse strain AU/ssJ (H-2q) which is resistant to collagen-induced arthritis. Immunogenetics. 1989;29(3):180–185. doi: 10.1007/BF00373643. [DOI] [PubMed] [Google Scholar]
- Haqqi T. M., David C. S. T-cell receptor V beta genes repertoire in mice. Possible role in resistance and susceptibility to type II collagen-induced arthritis. J Autoimmun. 1990 Apr;3(2):113–121. doi: 10.1016/0896-8411(90)90135-f. [DOI] [PubMed] [Google Scholar]
- Haworth C., Brennan F. M., Chantry D., Turner M., Maini R. N., Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur J Immunol. 1991 Oct;21(10):2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Bailey C., Enander I., Mayer R., Klareskog L., Moran T., Bona C. Origin of the autoreactive anti-type II collagen response. II. Specificities, antibody isotypes and usage of V gene families of anti-type II collagen B cells. J Immunol. 1989 Mar 15;142(6):1881–1886. [PubMed] [Google Scholar]
- Kim S. H., Yu S. S., Park J. S., Robbins P. D., An C. S., Kim S. Construction of retroviral vectors with improved safety, gene expression, and versatility. J Virol. 1998 Feb;72(2):994–1004. doi: 10.1128/jvi.72.2.994-1004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E., Joosten L. A., van Den Bersselaar L., Helsen M. M., Bakker A. C., van Meurs J. B., Graham F. L., Richards C. D., van Den Berg W. B. Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J Immunol. 1999 Oct 15;163(8):4546–4556. [PubMed] [Google Scholar]
- Luross J. A., Williams N. A. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001 Aug;103(4):407–416. doi: 10.1046/j.1365-2567.2001.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström V., Kjellén P., Holmdahl R. Type II collagen in cartilage evokes peptide-specific tolerance and skews the immune response. J Autoimmun. 1998 Jun;11(3):213–221. doi: 10.1006/jaut.1998.0198. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Baumgartner S. W., Schiff M. H., Tindall E. A., Fleischmann R. M., Weaver A. L., Ettlinger R. E., Cohen S., Koopman W. J., Mohler K. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997 Jul 17;337(3):141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Moudgil K. D., Chang T. T., Eradat H., Chen A. M., Gupta R. S., Brahn E., Sercarz E. E. Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. J Exp Med. 1997 Apr 7;185(7):1307–1316. doi: 10.1084/jem.185.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., Wu B., Mayton L., Kim S-H, Robbins P. D., Wooley P. H. TNF receptor gene therapy results in suppression of IgG2a anticollagen antibody in collagen induced arthritis. Ann Rheum Dis. 2003 Aug;62(8):707–714. doi: 10.1136/ard.62.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L. K., Rosloniec E. F., Cremer M. A., Kang A. H. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61(19):1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Myers L. K., Seyer J. M., Stuart J. M., Terato K., David C. S., Kang A. H. T cell epitopes of type II collagen that regulate murine collagen-induced arthritis. J Immunol. 1993 Jul 1;151(1):500–505. [PubMed] [Google Scholar]
- Nabozny G. H., Bull M. J., Hanson J., Griffiths M. M., Luthra H. S., David C. S. Collagen-induced arthritis in T cell receptor V beta congenic B10.Q mice. J Exp Med. 1994 Aug 1;180(2):517–524. doi: 10.1084/jem.180.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H., Hashim G. A., Vandenbark A. A. T cell receptor peptide therapy triggers autoregulation of experimental encephalomyelitis. Science. 1991 Jan 25;251(4992):430–432. doi: 10.1126/science.1989076. [DOI] [PubMed] [Google Scholar]
- Osman G. E., Toda M., Kanagawa O., Hood L. E. Characterization of the T cell receptor repertoire causing collagen arthritis in mice. J Exp Med. 1993 Feb 1;177(2):387–395. doi: 10.1084/jem.177.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K., Crawford D., Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991 Dec 1;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchi E., Walmsley M., Browne K., Williams R. O., Marinova-Mutafchieva L., Buurman W., Butler D. M., Feldmann M. Paradoxical effects of adenovirus-mediated blockade of TNF activity in murine collagen-induced arthritis. J Immunol. 1999 Jul 15;163(2):1000–1009. [PubMed] [Google Scholar]
- Rankin E. C., Choy E. H., Kassimos D., Kingsley G. H., Sopwith A. M., Isenberg D. A., Panayi G. S. The therapeutic effects of an engineered human anti-tumour necrosis factor alpha antibody (CDP571) in rheumatoid arthritis. Br J Rheumatol. 1995 Apr;34(4):334–342. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- Taylor P. C., Plater-Zyberk C., Maini R. N. The role of the B cells in the adoptive transfer of collagen-induced arthritis from DBA/1 (H-2q) to SCID (H-2d) mice. Eur J Immunol. 1995 Mar;25(3):763–769. doi: 10.1002/eji.1830250321. [DOI] [PubMed] [Google Scholar]
- Thorbecke G. J., Shah R., Leu C. H., Kuruvilla A. P., Hardison A. M., Palladino M. A. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlugt Carol L., Miller Stephen D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002 Feb;2(2):85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Somia N. Gene therapy -- promises, problems and prospects. Nature. 1997 Sep 18;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- Whalen J. D., Lechman E. L., Carlos C. A., Weiss K., Kovesdi I., Glorioso J. C., Robbins P. D., Evans C. H. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol. 1999 Mar 15;162(6):3625–3632. [PubMed] [Google Scholar]
- Wooley P. H., Dutcher J., Widmer M. B., Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J Immunol. 1993 Dec 1;151(11):6602–6607. [PubMed] [Google Scholar]
- Wooley P. H., Panayi G. S., Batchelor J. R. Lymphocytotoxins in rheumatoid arthritis: prevalence, lymphocyte specificity, and HLA-DR antigens. Ann Rheum Dis. 1981 Apr;40(2):154–156. doi: 10.1136/ard.40.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H., Schaefer C., Whalen J. D., Dutcher J. A., Counts D. F. A peptide sequence from platelet factor 4 (CT-112) is effective in the treatment of type II collagen induced arthritis in mice. J Rheumatol. 1997 May;24(5):890–898. [PubMed] [Google Scholar]
- Yoo T. J., Kim S. Y., Stuart J. M., Floyd R. A., Olson G. A., Cremer M. A., Kang A. H. Induction of arthritis in monkeys by immunization with type II collagen. J Exp Med. 1988 Aug 1;168(2):777–782. doi: 10.1084/jem.168.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]