Abstract
Objective: To determine whether oncostatin M (OSM) + tumour necrosis factor α (TNFα) induces aggrecanase activity in chondrocyte membranes, to determine the effects of transforming growth factor ß1 (TGFß1), interleukin 4 (IL4), and tissue inhibitor of metalloproteinases (TIMPs) on this activity, and to determine whether this activity is due to a known ADAMTS aggrecanase.
Methods: Aggrecanase activity and ability of agents to prevent membrane associated aggrecanase activity were assessed by Western blotting. Expression of known aggrecanases was measured by real time polymerase chain reaction in bovine nasal and human articular chondrocytes.
Results: Chondrocyte membrane associated aggrecanase activity and increased mRNA expression of ADAMTS-1, -4, -5, and -9, but not ADAMTS-4 or -15, were enhanced after stimulation by OSM+TNFα in bovine chondrocytes. This activity was inhibited by TIMP-3. In human chondrocytes, OSM+TNFα also enhanced ADAMTS-1 and -4 expression, but not that of other ADAMTSs. TNFα alone induced ADAMTS-9 expression, whereas OSM addition caused suppression. Both TGFß1 and IL4 blocked membrane associated aggrecanase activity and decreased OSM+TNFα-induced expression of ADAMTS-9 in bovine and human chondrocytes. IL4 down regulated ADAMTS-4 mRNA, whereas TGFß1 increased this expression in both bovine and human chondrocytes.
Conclusions: OSM+TNFα up regulates membrane associated aggrecanase activity and several ADAMTS aggrecanase mRNAs in chondrocytes. The chondroprotective effects of IL4 and TIMP-3 suggest that they may have therapeutic benefit for aggrecanolysis, whereas the differential inhibitory effects of TGFß1 may limit its therapeutic potential. Induced membrane associated aggrecanase activity is distinct from known soluble ADAMTS aggrecanases and merits further investigation.
Full Text
The Full Text of this article is available as a PDF (179.0 KB).
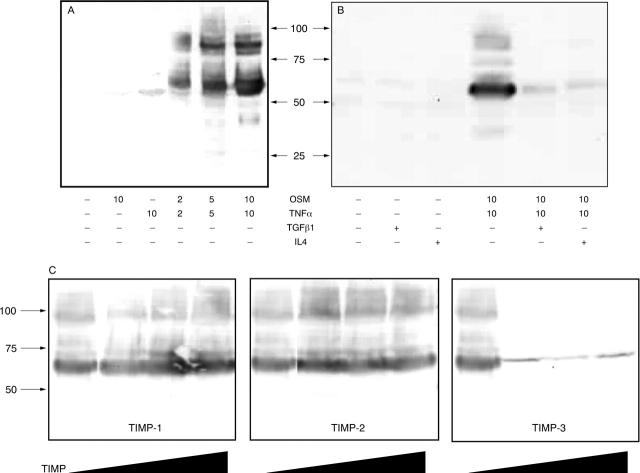
Figure 1.
Effect of cytokines and TIMPs on chondrocyte membrane associated aggrecanase activity. Bovine nasal chondrocytes were cultured until 80–90% confluence, serum starved overnight, and then stimulated with various cytokines individually or in combination for 24 hours. Harvested cells were lysed and the plasma membrane fraction enriched by density centrifugation. Membranes (50–100 µg) were incubated with deglycosylated aggrecan (100 µg) at 37°C for 16 hours. Aggrecanase activity was examined by Western blot using an antibody (R663) that recognises the neoepitope generated after cleavage of aggrecan at the Glu373–Ala374 bond. (A) Effect of cytokine dose on the membrane activity; (B) effect of TGFß1 (15 ng/ml) or IL4 (25 ng/ml) inclusion in the 24 hour chondrocyte stimulation; (C) the effect of TIMP-1, -2, and -3 (0.1, 0.5, or 1.5 µg), added for 30 minutes at room temperature before the addition of substrate. The data shown are representative of three separate chondrocyte preparations.
Figure 2.
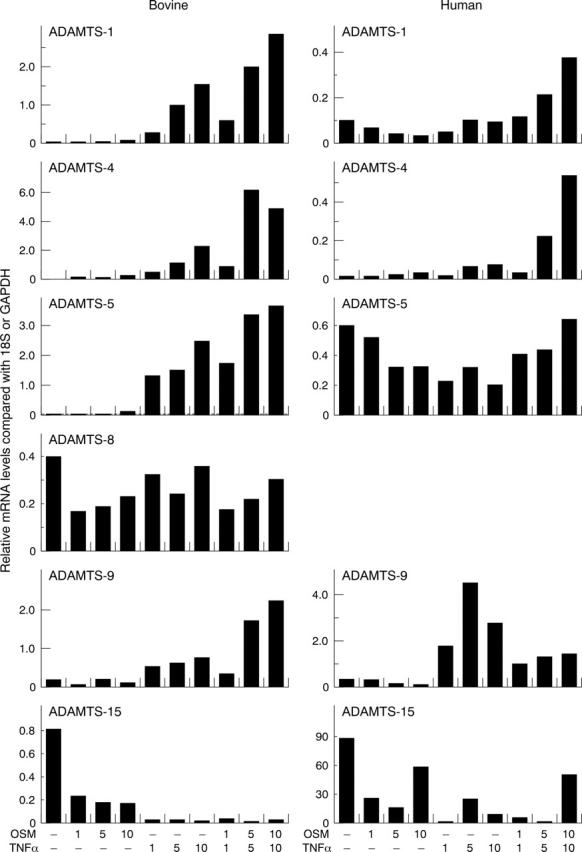
Effects of OSM and TNFα on the expression of ADAMTS aggrecanases in chondrocytes. Bovine nasal or human articular chondrocytes were cultured until 80–90% confluence and then serum starved overnight. Cells were stimulated with OSM, TNFα alone or in combination for 24 hours. Total RNA was isolated, reverse transcribed, and the resulting cDNA used in a separate real time PCR with specific primers for ADAMTS-1, -4, -5, -8, -9, or -15. No signal was detected for ADAMTS-8 in human chondrocytes with any treatment. Results were normalised to either 18S (for bovine) or GAPDH (for human) and are presented graphically as relative mRNA levels.48 The data shown are representative of two separate chondrocyte preparations.
Figure 3.
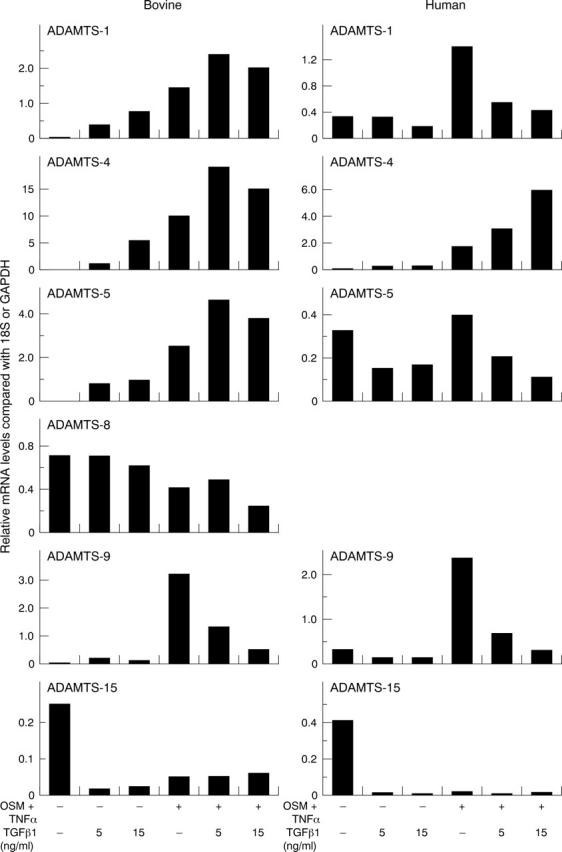
Effects of TGFß1 on OSM+TNFα-induced ADAMTS aggrecanases. Bovine nasal or human articular chondrocytes were stimulated with OSM+TNFα (both at 10 ng/ml)±TGFß1 for 24 hours. Real time PCR was performed on isolated RNA, and the normalised results presented graphically as described in fig 2. No signal was detected for ADAMTS-8 in human chondrocytes with any treatment. The data shown are representative of two separate chondrocyte preparations.
Figure 4.
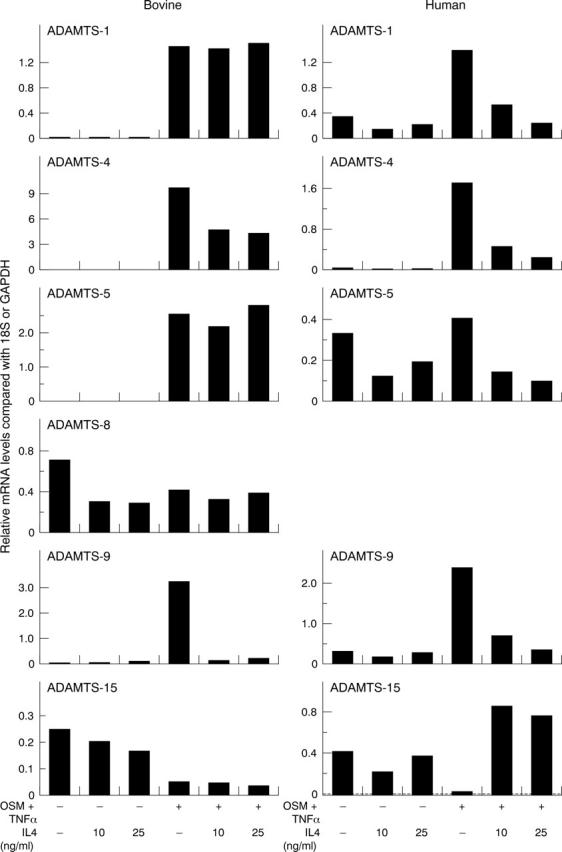
Effects of IL4 on OSM+TNFα-induced ADAMTS aggrecanases. Bovine nasal or human articular chondrocytes were stimulated with OSM+TNFα (both at 10 ng/ml) ± IL4 for 24 hours. Real time PCR was performed on isolated RNA, and the normalised results presented graphically as described in fig 2. No signal was detected for ADAMTS-8 in human chondrocytes with any treatment. The data shown are representative of two separate chondrocyte preparations.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszade I., Liu R. Q., Yang F., Rosenfeld S. A., Ross O. H., Link J. R., Ellis D. M., Tortorella M. D., Pratta M. A., Hollis J. M. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999 Aug 13;274(33):23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Arner E. C., Pratta M. A., Trzaskos J. M., Decicco C. P., Tortorella M. D. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999 Mar 5;274(10):6594–6601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- Arris Christine E., Bevitt Debra J., Mohamed Jeseem, Li Zheng, Langton Kevin P., Barker Michael D., Clarke Michael P., McKie Norman. Expression of mutant and wild-type TIMP3 in primary gingival fibroblasts from Sorsby's fundus dystrophy patients. Biochim Biophys Acta. 2003 May 20;1638(1):20–28. doi: 10.1016/s0925-4439(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Bau Brigitte, Gebhard Pia M., Haag Jochen, Knorr Thomas, Bartnik Eckart, Aigner Thomas. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002 Oct;46(10):2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Billington C. J., Clark I. M., Cawston T. E. An aggrecan-degrading activity associated with chondrocyte membranes. Biochem J. 1998 Nov 15;336(Pt 1):207–212. doi: 10.1042/bj3360207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluteau G., Conrozier T., Mathieu P., Vignon E., Herbage D., Mallein-Gerin F. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta. 2001 May 3;1526(2):147–158. doi: 10.1016/s0304-4165(01)00122-2. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Browne K. A., Green P. A., Jaspar J. M., Maini R. N., Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-alpha (cA2) therapy. Br J Rheumatol. 1997 Jun;36(6):643–650. doi: 10.1093/rheumatology/36.6.643. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Fowles A., Ilic M. Z., Handley C. J. "Aggrecanase" activity is implicated in tumour necrosis factor alpha mediated cartilage aggrecan breakdown but is not detected by an in vitro assay. Mol Pathol. 1997 Jun;50(3):153–159. doi: 10.1136/mp.50.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Curry V. A., Summers C. A., Clark I. M., Riley G. P., Life P. F., Spaull J. R., Goldring M. B., Koshy P. J., Rowan A. D. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998 Oct;41(10):1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cawston T., Billington C., Cleaver C., Elliott S., Hui W., Koshy P., Shingleton B., Rowan A. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999 Jun 30;878:120–129. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- Clark M. E., Kelner G. S., Turbeville L. A., Boyer A., Arden K. C., Maki R. A. ADAMTS9, a novel member of the ADAM-TS/ metallospondin gene family. Genomics. 2000 Aug 1;67(3):343–350. doi: 10.1006/geno.2000.6246. [DOI] [PubMed] [Google Scholar]
- Cleaver C. S., Rowan A. D., Cawston T. E. Interleukin 13 blocks the release of collagen from bovine nasal cartilage treated with proinflammatory cytokines. Ann Rheum Dis. 2001 Feb;60(2):150–157. doi: 10.1136/ard.60.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Racie Lisa A., Flannery Carl R., Zeng Weilan, Corcoran Chris, Annis-Freeman Bethany, Agostino Michael J., Arai Maya, DiBlasio-Smith Elizabeth, Dorner Andrew J., Georgiadis Katy E. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004 Jul;23(4):219–230. doi: 10.1016/j.matbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Curry V. A., Clark I. M., Bigg H., Cawston T. E. Large inhibitor of metalloproteinases (LIMP) contains tissue inhibitor of metalloproteinases (TIMP)-2 bound to 72,000-M(r) progelatinase. Biochem J. 1992 Jul 1;285(Pt 1):143–147. doi: 10.1042/bj2850143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Dingle T. T. The site of cartilage matrix degradation. Biochem J. 1980 Aug 15;190(2):431–438. doi: 10.1042/bj1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery C. R., Little C. B., Hughes C. E., Curtis C. L., Caterson B., Jones S. A. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol. 2000 Nov;19(6):549–553. doi: 10.1016/s0945-053x(00)00111-6. [DOI] [PubMed] [Google Scholar]
- Gao Gui, Plaas Anna, Thompson Vivian P., Jin Sue, Zuo Fengrong, Sandy John D. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2003 Dec 29;279(11):10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- Gao Gui, Westling Jennifer, Thompson Vivian P., Howell Troy D., Gottschall Paul E., Sandy John D. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002 Jan 16;277(13):11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- Gendron Christi, Kashiwagi Masahide, Hughes Clare, Caterson Bruce, Nagase Hideaki. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett. 2003 Dec 18;555(3):431–436. doi: 10.1016/s0014-5793(03)01295-x. [DOI] [PubMed] [Google Scholar]
- Grimaud Eva, Heymann Dominique, Rédini Françoise. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002 Jun;13(3):241–257. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hashimoto G., Aoki T., Nakamura H., Tanzawa K., Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett. 2001 Apr 13;494(3):192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Hodges D. J., Reid D. G., Rowan A. D., Clark I. M., Cawston T. E. Preparation of recombinant tissue inhibitor of metalloproteinases-1 (TIMP-1) in high yield and identification of a hydrophobic surface feature. Eur J Biochem. 1998 Nov 1;257(3):562–569. doi: 10.1046/j.1432-1327.1998.2570562.x. [DOI] [PubMed] [Google Scholar]
- Hui W., Cawston T., Rowan A. D. Transforming growth factor beta 1 and insulin-like growth factor 1 block collagen degradation induced by oncostatin M in combination with tumour necrosis factor alpha from bovine cartilage. Ann Rheum Dis. 2003 Feb;62(2):172–174. doi: 10.1136/ard.62.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Rowan A. D., Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine. 2001 Oct 7;16(1):31–35. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- Hui W., Rowan A. D., Cawston T. Transforming growth factor beta1 blocks the release of collagen fragments from boving nasal cartilage stimulated by oncostatin M in combination with IL-1alpha. Cytokine. 2000 Jun;12(6):765–769. doi: 10.1006/cyto.1999.0625. [DOI] [PubMed] [Google Scholar]
- Hui W., Rowan A. D., Richards C. D., Cawston T. E. Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum. 2003 Dec;48(12):3404–3418. doi: 10.1002/art.11333. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M., Tortorella M., Nagase H., Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001 Jan 23;276(16):12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Kevorkian Lara, Young David A., Darrah Clare, Donell Simon T., Shepstone Lee, Porter Sarah, Brockbank Sarah M. V., Edwards Dylan R., Parker Andrew E., Clark Ian M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004 Jan;50(1):131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Koshy P. J. T., Lundy C. J., Rowan A. D., Porter S., Edwards D. R., Hogan A., Clark I. M., Cawston T. E. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002 Apr;46(4):961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Koshy P. J., Henderson N., Logan C., Life P. F., Cawston T. E., Rowan A. D. Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokines. Ann Rheum Dis. 2002 Aug;61(8):704–713. doi: 10.1136/ard.61.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark M. W., Bayne E. K., Flanagan J., Harper C. F., Hoerrner L. A., Hutchinson N. I., Singer I. I., Donatelli S. A., Weidner J. R., Williams H. R. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997 Jul 1;100(1):93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little Christopher B., Hughes Clare E., Curtis Clare L., Janusz Mike J., Bohne Richard, Wang-Weigand Sherry, Taiwo Yetunde O., Mitchell Peter G., Otterness Ivan G., Flannery Carl R. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002 Apr;21(3):271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Stanley K. K. The isolation of endosome-derived vesicles from rat hepatocytes. Biochem J. 1983 Oct 15;216(1):27–36. doi: 10.1042/bj2160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait Anne-Marie, Liu Rui-Qin, Ijiri Kosei, Komiya Setsuro, Tortorella Micky D. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002 Apr 15;277(25):22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Poilvache P., Van Egeren A., Devogelaer J. P., Lenz M. E., Thonar E. J. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000 Feb;43(2):281–288. doi: 10.1002/1529-0131(200002)43:2<281::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Milner J. M., Rowan A. D., Elliott S-F, Cawston T. E. Inhibition of furin-like enzymes blocks interleukin-1alpha/oncostatin M-stimulated cartilage degradation. Arthritis Rheum. 2003 Apr;48(4):1057–1066. doi: 10.1002/art.10873. [DOI] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Mineau F., Dupuis M., Cloutier J. M., Martel-Pelletier J. Modulation of collagenase 3 in human osteoarthritic cartilage by activation of extracellular transforming growth factor beta: role of furin convertase. Arthritis Rheum. 2000 Sep;43(9):2100–2109. doi: 10.1002/1529-0131(200009)43:9<2100::AID-ANR22>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Morales T. I., Hascall V. C. Correlated metabolism of proteoglycans and hyaluronic acid in bovine cartilage organ cultures. J Biol Chem. 1988 Mar 15;263(8):3632–3638. [PubMed] [Google Scholar]
- Moulharat N., Lesur C., Thomas M., Rolland-Valognes G., Pastoureau P., Anract P., De Ceuninck F., Sabatini M. Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage. 2004 Apr;12(4):296–305. doi: 10.1016/j.joca.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Nagase Hideaki, Kashiwagi Masahide. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003 Feb 14;5(2):94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C., Buckton J., Thompson S., Spaull J., Zanders E., Papworth J., Life P. F. Amelioration of arthritis in two murine models using antibodies to oncostatin M. Arthritis Rheum. 2001 Nov;44(11):2697–2702. doi: 10.1002/1529-0131(200111)44:11<2697::aid-art450>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Poole A. Robin, Nelson Fred, Dahlberg Leif, Tchetina Elena, Kobayashi Masahiko, Yasuda Tadashi, Laverty Sheila, Squires Ginette, Kojima Toshihisa, Wu William. Proteolysis of the collagen fibril in osteoarthritis. Biochem Soc Symp. 2003;(70):115–123. doi: 10.1042/bss0700115. [DOI] [PubMed] [Google Scholar]
- Porter Sarah, Clark Ian M., Kevorkian Lara, Edwards Dylan R. The ADAMTS metalloproteinases. Biochem J. 2005 Feb 15;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratta Michael A., Scherle Peggy A., Yang Gengjie, Liu Rui-Qin, Newton Robert C. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003 Jan;48(1):119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzaneque Juan Carlos, Westling Jennifer, Thai Shelley N-M, Luque Alfonso, Knauper Vera, Murphy Gillian, Sandy John D., Iruela-Arispe M. Luisa. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002 Apr 26;293(1):501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Rowan Andrew D., Hui Wang, Cawston Tim E., Richards Carl D. Adenoviral gene transfer of interleukin-1 in combination with oncostatin M induces significant joint damage in a murine model. Am J Pathol. 2003 Jun;162(6):1975–1984. doi: 10.1016/S0002-9440(10)64330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001 Sep 15;358(Pt 3):615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville Robert P. T., Longpre Jean-Michel, Jungers Katherine A., Engle J. Michael, Ross Monique, Evanko Stephen, Wight Thomas N., Leduc Richard, Apte Suneel S. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003 Jan 3;278(11):9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Su S., Grover J., Roughley P. J., DiBattista J. A., Martel-Pelletier J., Pelletier J. P., Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18(5-6):183–191. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- Sztrolovics Robert, Recklies Anneliese D., Roughley Peter J., Mort John S. Hyaluronate degradation as an alternative mechanism for proteoglycan release from cartilage during interleukin-1beta-stimulated catabolism. Biochem J. 2002 Mar 1;362(Pt 2):473–479. doi: 10.1042/0264-6021:3620473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztrolovics Robert, White Robert J., Roughley Peter J., Mort John S. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002 Mar 1;362(Pt 2):465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella M. D., Burn T. C., Pratta M. A., Abbaszade I., Hollis J. M., Liu R., Rosenfeld S. A., Copeland R. A., Decicco C. P., Wynn R. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999 Jun 4;284(5420):1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Tortorella M., Pratta M., Liu R. Q., Abbaszade I., Ross H., Burn T., Arner E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000 Aug 18;275(33):25791–25797. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke M. N., Holen I., Wilson A. G., Ilic M. Z., Handley C. J., Kelner G. S., Clark M., Liu C., Maki R. A., Burnett D. Expression and activity of ADAMTS-5 in synovium. Eur J Biochem. 2001 Mar;268(5):1259–1268. doi: 10.1046/j.1432-1327.2001.01990.x. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke M. N., Ilic M. Z., Handley C. J., Knight C. G., Buttle D. J. Coincubation of bovine synovial or capsular tissue with cartilage generates a soluble "Aggrecanase" activity. Biochem Biophys Res Commun. 1999 Feb 24;255(3):686–691. doi: 10.1006/bbrc.1999.0266. [DOI] [PubMed] [Google Scholar]
- Vázquez F., Hastings G., Ortega M. A., Lane T. F., Oikemus S., Lombardo M., Iruela-Arispe M. L. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999 Aug 13;274(33):23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Yamanishi Yuji, Boyle David L., Clark Melody, Maki Rich A., Tortorella Micky D., Arner Elizabeth C., Firestein Gary S. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002 Feb 1;168(3):1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- van Lent P. L. E. M., Holthuysen A. E. M., Slöetjes A., Lubberts E., van den Berg W. B. Local overexpression of adeno-viral IL-4 protects cartilage from metallo proteinase-induced destruction during immune complex-mediated arthritis by preventing activation of pro-MMPs. Osteoarthritis Cartilage. 2002 Mar;10(3):234–243. doi: 10.1053/joca.2001.0501. [DOI] [PubMed] [Google Scholar]