Abstract
Patients and methods: A prospective, multicentre, randomised, double blind phase 4 study of 355 patients with RA in two groups: group A received betamethasone and group B placebo, before a 36 week infusion treatment with infliximab. Incidence and severity of infusion reactions from infliximab treatment were assessed.
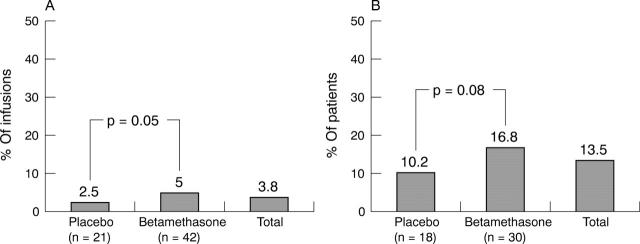
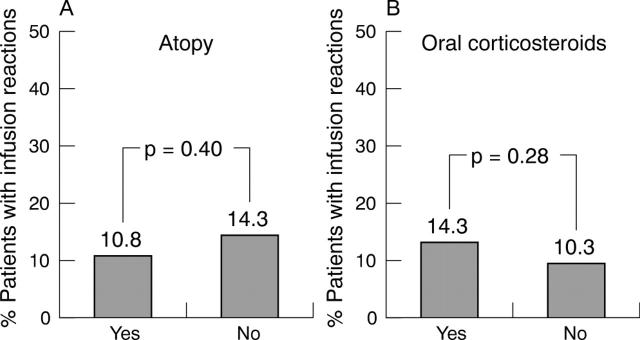
Results: The incidence of reactions to infliximab infusion was <5%. More infusion reactions occurred with betamethasone pretreatment than with placebo. Response to infliximab of patients with atopic backgrounds did not differ in the presence or absence of betamethasone from that of non-atopic patients. Mean Health Assessment Questionnaire score improved by 47% at week 24, quality of life assessed by Short Form-36 improved in mental and physical component subscales.
Conclusions: Incidence of infusion reactions with infliximab was low and their severity generally mild, but betamethasone pretreatment did not decrease the incidence and severity of infusion reactions. Betamethasone, therefore, is not recommended as a systematic prophylactic measure, even in atopic patients.
Full Text
The Full Text of this article is available as a PDF (52.1 KB).
Figure 1.
(A) Percentage of infusions in which infusion reactions occurred. (B) Percentage of patients in whom infusion reactions occurred.
Figure 2.
Percentage of patients in whom infusion reactions occurred in the presence or absence of (A) an atopic background and (B) the use of oral corticosteroids.