Abstract
Background: Systemic lupus erythematosus (SLE) is an autoimmune disease affecting multiple organ systems triggered by the production of autoantibodies. Previous clinical studies in humans and murine models suggest that type I interferons (IFNs) are important for the initiation and potentiation of SLE activity.
Methods: 65 consecutive patients with SLE were identified from the University of California, San Francisco Lupus Clinic with moderate-severe disease activity. 94 serological samples were collected. Type I IFN levels and the ability of plasma to induce expression of several surface markers of dendritic cell maturation were measured.
Results: Type I IFN levels correlated with the presence of cutaneous manifestations, and there was a trend towards correlation with renal disease. No correlation was found between type I IFN levels and neurological disease. Type I IFN levels correlated positively with the SLEDAI score and anti-dsDNA levels and inversely with C3 levels. Interestingly, type I IFN levels were highest in African American patients. SLE plasma also induced the expression of MHC class I, CD38, and CD123 on monocytes, and was blocked by the addition of a monoclonal antibody to IFNAR1.
Conclusions: The pathogenic role of type I IFN is suggested by the induction of cell surface markers for dendritic cell maturation. The potential therapeutic utility of antibodies directed to either type I IFN or IFNAR1/IFNAR2 may be of interest in further studies.
Full Text
The Full Text of this article is available as a PDF (117.8 KB).
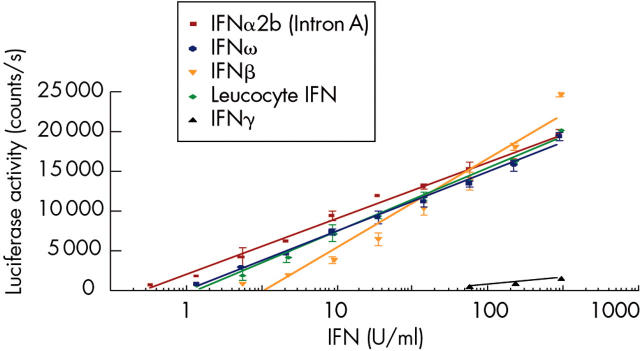
Figure 1.
The human hepatoma cell line Hil3, transfected with ISRE-Luc, responds to type I IFN stimulation in a dose dependent manner. Luciferase activity is measured in counts per second and is proportional to IFN activity as demonstrated by linear regression analysis (Graphpad Prism). All recombinant type I IFN subtypes tested show significant activity. Similarly, natural leucocyte IFN (multiple IFNα subtypes + IFNω) produces a robust luciferase response. In contrast, the Hil3 reporter assay exhibits a very weak response to type II IFN (IFNγ).
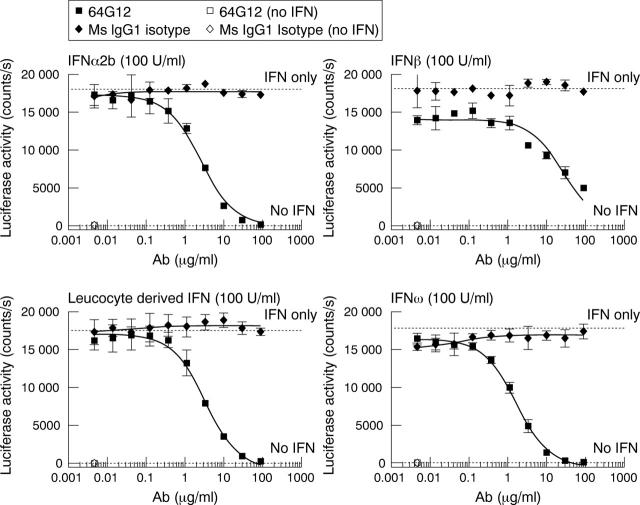
Figure 2.
Type I IFN activity in the Hil3 ISRE-Luc reporter assay is neutralised by mouse anti-IFNAR1 (64G12) in a dose dependent manner. A fixed concentration of IFN (100 U/ml) is added to cells in the presence of a dose titration of IFNAR1 specific antibody or isotype control. Luciferase activity is measured in counts per second and neutralisation is proportional to antibody concentration as demonstrated by non-linear regression analysis, sigmoidal dose response, variable slope (Graphpad Prism).
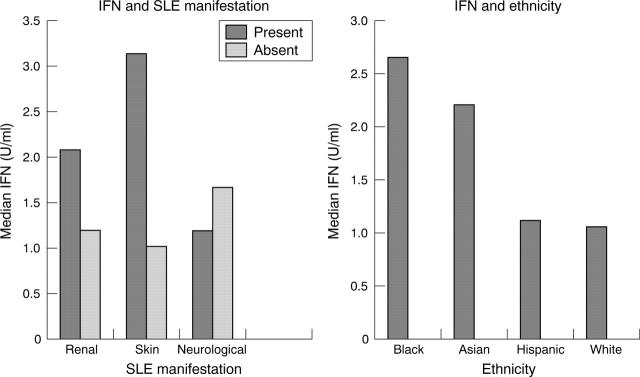
Figure 3.
IFN correlated significantly with the presence of skin disease (median 3.138 U/ml v 1.022 U/ml, p = 0.0002). There was a trend towards a positive correlation between IFN and the presence of renal disease (median 2.072 U/ml v 1.193 U/ml, p = 0.0669). No statistically significant correlation between IFN and neurological disease was found. IFN levels were significantly higher in African American patients than in white patients (median 2.651 U/ml v 1.060 U/ml, p = 0.0299).
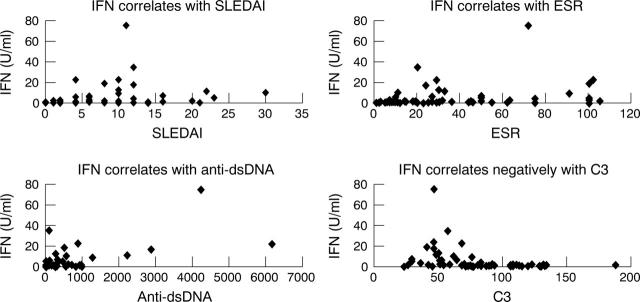
Figure 4.
IFN levels correlated positively with SLEDAI scores (rs = 0.451, p = 0.0002), ESR (rs = 0.481, p = 0.0002), and anti-dsDNA (rs = 0.509, p = 0.000). IFN levels correlated negatively with C3 levels (rs = –0.591, p = 0.000).
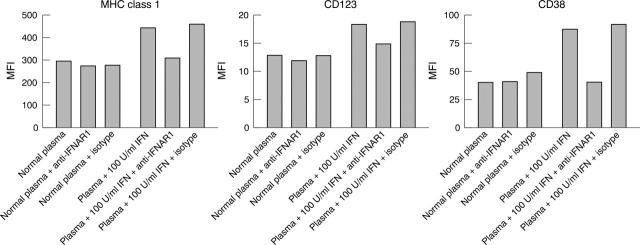
Figure 5.
Anti-IFNAR blockade of recombinant IFNα mediated dendritic cell development. Monocyte differentiation was monitored by increased expression of the cell surface markers MHC class I, CD123, and CD38. Normal healthy donor plasma does not mediate IFN dependent changes in monocyte maturation as demonstrated by treatment of monocytes in the presence and absence of blocking antibody. Normal plasma supplemented with 100 U/ml recombinant IFNα2b mediates an induction of marker expression in comparison with normal plasma alone. Neutralising mouse anti-IFNAR1 (64G12) returns the levels of MHC class I, CD123, and CD38 to baseline but has no effect on marker levels in the presence of normal plasma alone. Isotype control has no significant effect on marker levels.
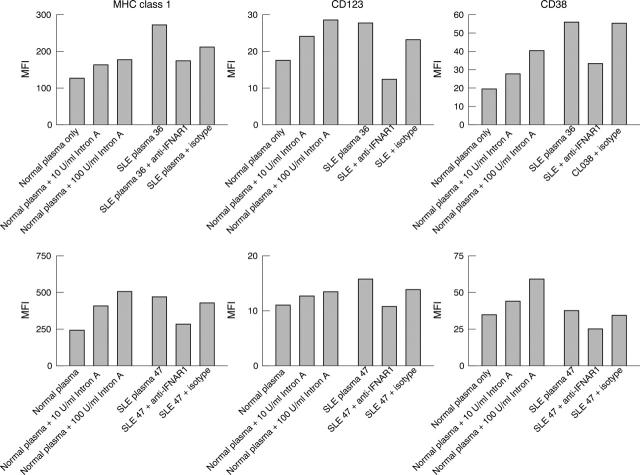
Figure 6.
Anti-IFNAR blockade of SLE plasma mediated DC development. Monocytes isolated from healthy donor PBMCs differentiate in response to IFN stimulation. This maturation process is monitored by up regulation in the expression of cell surface markers MHC class I, CD123, and CD38 after treatment of monocytes with normal plasma supplemented with 100 U/ml and 10 U/ml IFNα2b. Similarly, plasma from patients with SLE, in the absence of antibody, mediates induction of marker expression consistent with the activity of the type I IFN detected in a reporter assay. SLE plasma activity is neutralised in the presence of mouse anti-IFNAR1 (64G12), resulting in normalised MHC class I, CD123, and CD38 levels comparable to those of unstimulated monocytes. Isotype control has negligible effect on monocyte differentiation in the presence of SLE plasma.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baechler Emily C., Batliwalla Franak M., Karypis George, Gaffney Patrick M., Ortmann Ward A., Espe Karl J., Shark Katherine B., Grande William J., Hughes Karis M., Kapur Vivek. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003 Feb 25;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson A. A., Sturfelt G., Truedsson L., Blomberg J., Alm G., Vallin H., Rönnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9(9):664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- Benoit P., Maguire D., Plavec I., Kocher H., Tovey M., Meyer F. A monoclonal antibody to recombinant human IFN-alpha receptor inhibits biologic activity of several species of human IFN-alpha, IFN-beta, and IFN-omega. Detection of heterogeneity of the cellular type I IFN receptor. J Immunol. 1993 Feb 1;150(3):707–716. [PubMed] [Google Scholar]
- Blanco P., Palucka A. K., Gill M., Pascual V., Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001 Nov 16;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Braun Déborah, Geraldes Pedro, Demengeot Jocelyne. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003 Feb;20(1):15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Preble O., Black R., Harrell S. Interferon production in patients with systemic lupus erythematosus. Arthritis Rheum. 1982 Jul;25(7):802–803. doi: 10.1002/art.1780250717. [DOI] [PubMed] [Google Scholar]
- Han G-M, Chen S-L, Shen N., Ye S., Bao C-D, Gu Y-Y. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003 Apr;4(3):177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- Hochberg M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997 Sep;40(9):1725–1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- Hooks J. J., Moutsopoulos H. M., Geis S. A., Stahl N. I., Decker J. L., Notkins A. L. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979 Jul 5;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Ioannou Y., Isenberg D. A. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000 Jul;43(7):1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kim T., Kanayama Y., Negoro N., Okamura M., Takeda T., Inoue T. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol. 1987 Dec;70(3):562–569. [PMC free article] [PubMed] [Google Scholar]
- Pogue Sarah L., Preston Benjamin T., Stalder Joseph, Bebbington Christopher R., Cardarelli Pina M. The receptor for type I IFNs is highly expressed on peripheral blood B cells and monocytes and mediates a distinct profile of differentiation and activation of these cells. J Interferon Cytokine Res. 2004 Feb;24(2):131–139. doi: 10.1089/107999004322813372. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Black R. J., Friedman R. M., Klippel J. H., Vilcek J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982 Apr 23;216(4544):429–431. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- Rönnblom Lars, Alm Gunnar V. Systemic lupus erythematosus and the type I interferon system. Arthritis Res Ther. 2003 Jan 20;5(2):68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Raber Marie-Laure, Baccala Roberto, Haraldsson Katarina M., Choubey Divaker, Stewart Timothy A., Kono Dwight H., Theofilopoulos Argyrios N. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003 Mar 17;197(6):777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling P. J., Kurzrock R., Kantarjian H., Gutterman J. U., Talpaz M. Development of systemic lupus erythematosus after interferon therapy for chronic myelogenous leukemia. Cancer. 1991 Oct 1;68(7):1536–1537. doi: 10.1002/1097-0142(19911001)68:7<1536::aid-cncr2820680713>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Slavikova Monika, Schmeisser Hana, Kontsekova Eva, Mateicka Frantisek, Borecky Ladislav, Kontsek Peter. Incidence of autoantibodies against type I and type II interferons in a cohort of systemic lupus erythematosus patients in Slovakia. J Interferon Cytokine Res. 2003 Mar;23(3):143–147. doi: 10.1089/107999003321532475. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Yee A. M., Yip Y. K., Fischer H. D., Buyon J. P. Serum activity that confers acid lability to alpha-interferon in systemic lupus erythematosus: its association with disease activity and its independence from circulating alpha-interferon. Arthritis Rheum. 1990 Apr;33(4):563–568. doi: 10.1002/art.1780330414. [DOI] [PubMed] [Google Scholar]
- Ytterberg S. R., Schnitzer T. J. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982 Apr;25(4):401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- von Wussow P., Jakschies D., Hartung K., Deicher H. Presence of interferon and anti-interferon in patients with systemic lupus erythematosus. Rheumatol Int. 1988;8(5):225–230. doi: 10.1007/BF00269199. [DOI] [PubMed] [Google Scholar]